Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider two oppositely charged atoms with charges of +1 and -1 units, respectively. The two atoms interact with each other through electrostatic (Coulombic) and
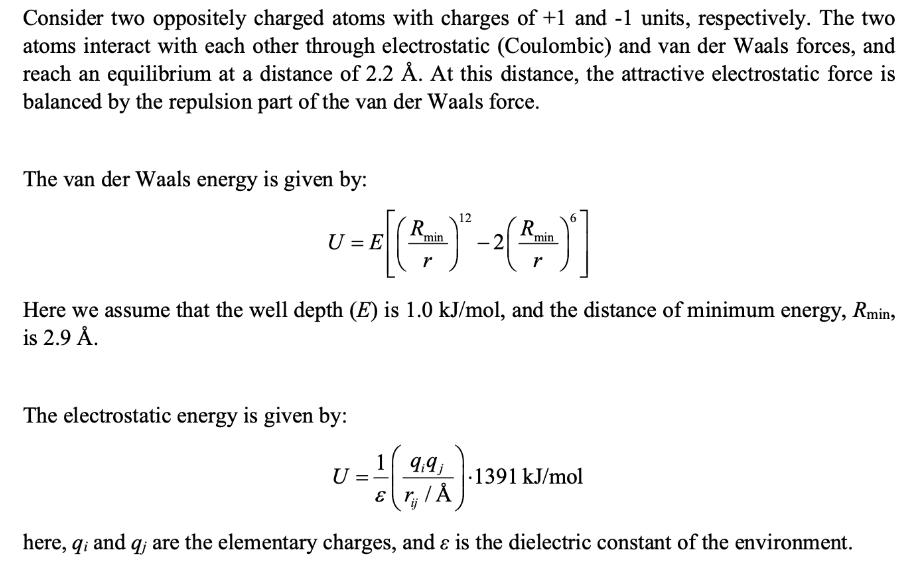

Consider two oppositely charged atoms with charges of +1 and -1 units, respectively. The two atoms interact with each other through electrostatic (Coulombic) and van der Waals forces, and reach an equilibrium at a distance of 2.2 . At this distance, the attractive electrostatic force is balanced by the repulsion part of the van der Waals force. The van der Waals energy is given by: R U=E min r 12 R 2 min r Here we assume that the well depth (E) is 1.0 kJ/mol, and the distance of minimum energy, Rmin, is 2.9 . The electrostatic energy is given by: 199 U .1391 kJ/mol here, q; and q; are the elementary charges, and & is the dielectric constant of the environment. (i) Derive an expression for the van der Waals force on one atom, starting from the energy equation given above. (ii) Derive an expression for the electrostatic force on one atom, starting from the energy equation given above. (iii) Calculate the value of , the dielectric constant, given that the van der Waals repulsion and the electrostatic attraction are balanced at 2.2 . Based on your answer, do you think that the environment is (a) water, (b) interior of a protein, or (c) vacuum?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


