Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider what kind of measurements are required to estimate a density (the mass and volume of your sample must be determined), as well as the
Consider what kind of measurements are required to estimate a density (the mass and volume of your sample must be determined), as well as the process you should use to obtain that data. you have to use this website for the measurements: chemcollective Identifying an Unknown Liquid from its Density (cmu.edu)
i really need help with this question it's in two parts if you can draw the same table and plug the stuff that will be great thank you !
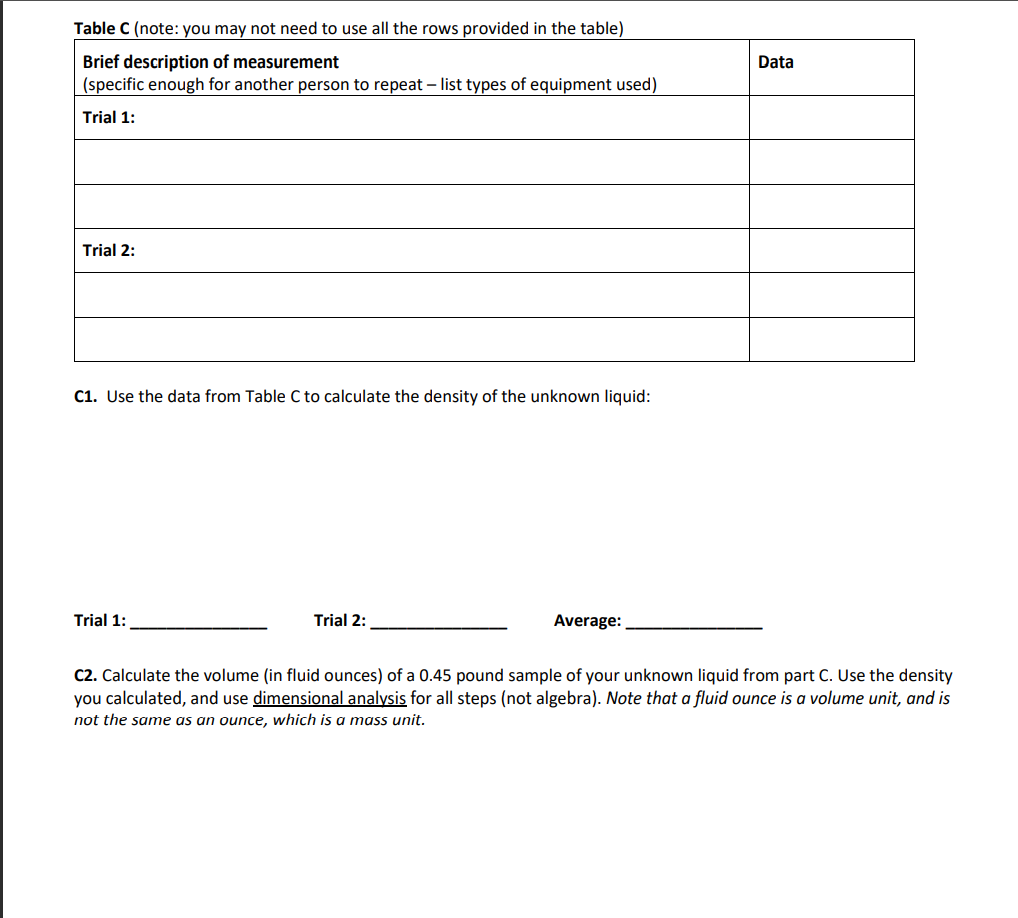
Table C (note: you may not need to use all the rows provided in the table) Brief description of measurement (specific enough for another person to repeat - list types of equipment used) Data Trial 1: Trial 2: C1. Use the data from Table C to calculate the density of the unknown liquid: Trial 1: Trial 2: Average: C2. Calculate the volume (in fluid Ounces) of a 0.45 pound sample of your unknown liquid from part C. Use the density you calculated, and use dimensional analysis for all steps (not algebra). Note that a fluid ounce is a volume unit, and is not the same as an ounce, which is a mass unit. Table C (note: you may not need to use all the rows provided in the table) Brief description of measurement (specific enough for another person to repeat - list types of equipment used) Data Trial 1: Trial 2: C1. Use the data from Table C to calculate the density of the unknown liquid: Trial 1: Trial 2: Average: C2. Calculate the volume (in fluid Ounces) of a 0.45 pound sample of your unknown liquid from part C. Use the density you calculated, and use dimensional analysis for all steps (not algebra). Note that a fluid ounce is a volume unit, and is not the same as an ounce, which is a mass unitStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


