Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Considering pure water at 15.6 C, determine the density and bulk modulus from Table 1.6. From Appendix C, determine the value of standard atmospheric pressure
Considering pure water at 15.6 °C, determine the density and bulk modulus from Table 1.6.
From Appendix C, determine the value of standard atmospheric pressure at sea level elevation. Using both
results, estimate a) density and b) specific gravity of liquid water at 15.6 °C and 1000 atmospheres of pressure
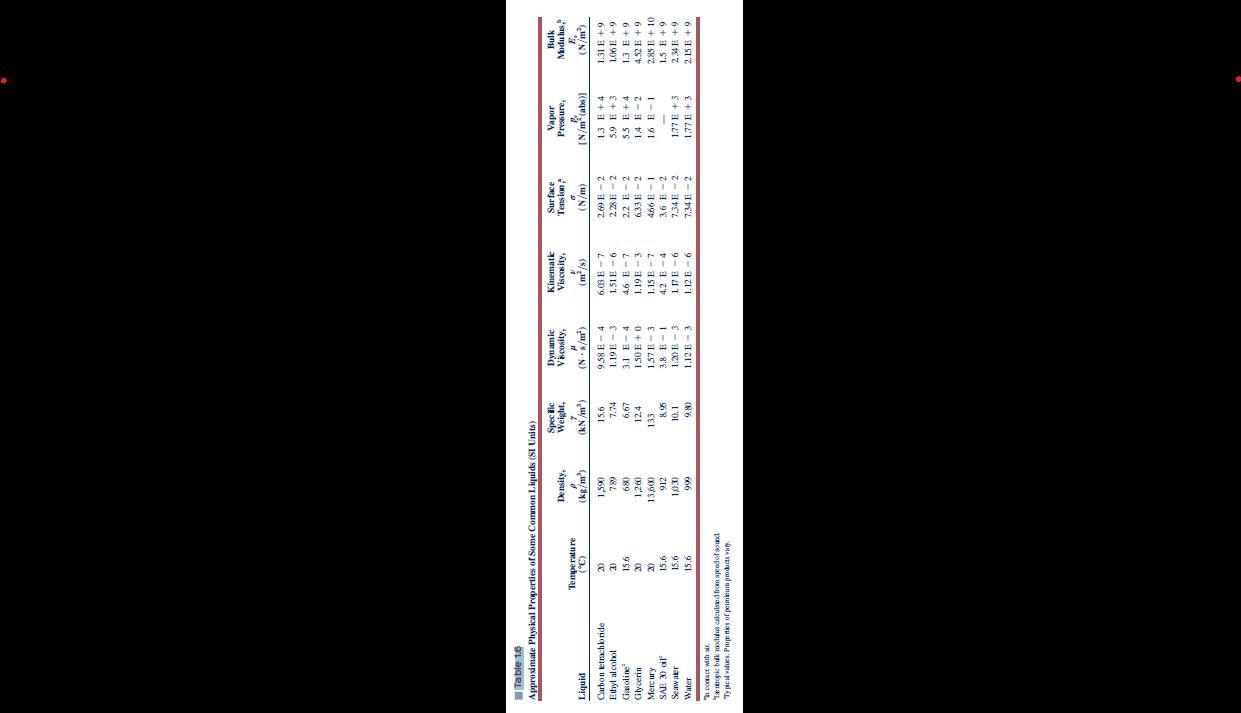
Image transcription text
Table 1.6 Approximate Physical Properties of Some Common Liquids (SI Units) Specific Dynamic Kinemati Surface Vapor Bulk Density, Weight,Viscosity, Viscosity, Tension, Pressure, Modulus.& Tempe rature E. Liquid ('9) (kg/m') (kN /m') (N . s/m) (m /s) (N/m) [ N/m (abs)] (N/m)
Carbon etrach bride 1,590 15.6 9.58 E - 4 6.03 E - 7 2,69 E - 2 13 E + 4 1.31 E + 9 Ethyl alcohol 789 7.74 1.19E - 3 1.51E - 6 23E -2 5...
Table 1.6 Approximate Physical Properties of Some Common Liquids (SI Units) Density, Specific Weight, Dynamic Viscosity, Kinematic Viscosity, Surface Tension," Temperature 7 0 Liquid (C) (kg/m) (kN/m) (N-8/m) (m/s) (N/m) Carbon tetrachloride 20 1,590 15.6 9.58 E-4 Ethyl alcohol 2) 789 7.74 1.19E 3 Gasoline 15.6 680 6.67 3.1 E-4 Glycerin 20 1,260 12.4 1.50E+0 Mercury 20 13,600 133 1.57B-3 6.03 E-7 1.51E-6 4.6 E-7 1.19E 3 1.15 E 7 2.69 E-2 228B-2 2.2 E-2 633B-2 5.9 E +3 55 E+4 Vapor Pressure, [N/m(abs)] 13 E+4 Bulk Modulus, E, (N/m) 1.31 E +9 106E +9 1.3 E+9 14 E-2 4.52 +9 466 E-1 16 E-1 SAE 30 oil 15.6 912 8.95 3.8 E 1 4.2 E-4 36 E-2 Seawater 15.6 1,030 10.1 1.20 E-3 117E-6 7.34 E-2 1.77 E + 3 2.85 E+10 1.5 E+9 234E +9 Water 15.6 999 9.80 1.12 E 3 1.12 E 6 7.34 B-2 1.77 B+3 2.15 E+9 " contact with air. Tentropic bulk modulus calculated from speed of sound Typical values. Properties of petroleum products vary.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To determine the density and bulk modulus of pure water at 156 C we first find the values from Table ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


