Answered step by step
Verified Expert Solution
Question
1 Approved Answer
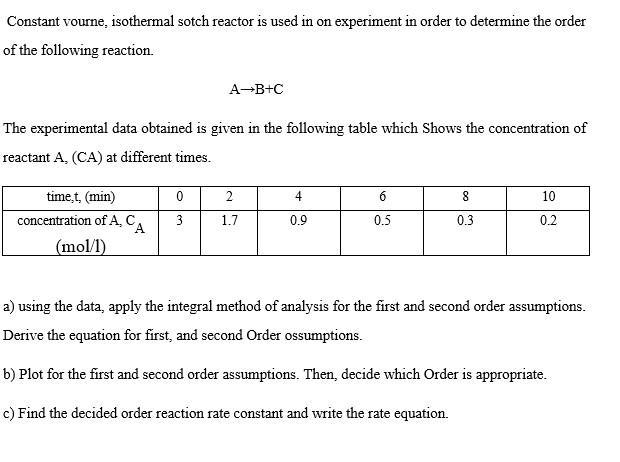
Constant volume, isothermal batch reactor is used in experiment in order to determine the order of the following reaction. A B + C The experimental
Constant volume, isothermal batch reactor is used in experiment in order to determine the order
of the following reaction.
The experimental data obtained is given in the following table which Shows the concentration of
reactant at different times.
a using the data, apply the integral method of analysis for the first and second order assumptions.
Derive the equation for first, and second order assumptions.
b Plot for the first and second order assumptions. Then, decide which Order is appropriate.
c Find the decided order reaction rate constant and write the rate equation.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


