Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Transitions between energy levels in atoms or molecules depend upon the satisfaction of selection rules. The strength for a transition from an initial state
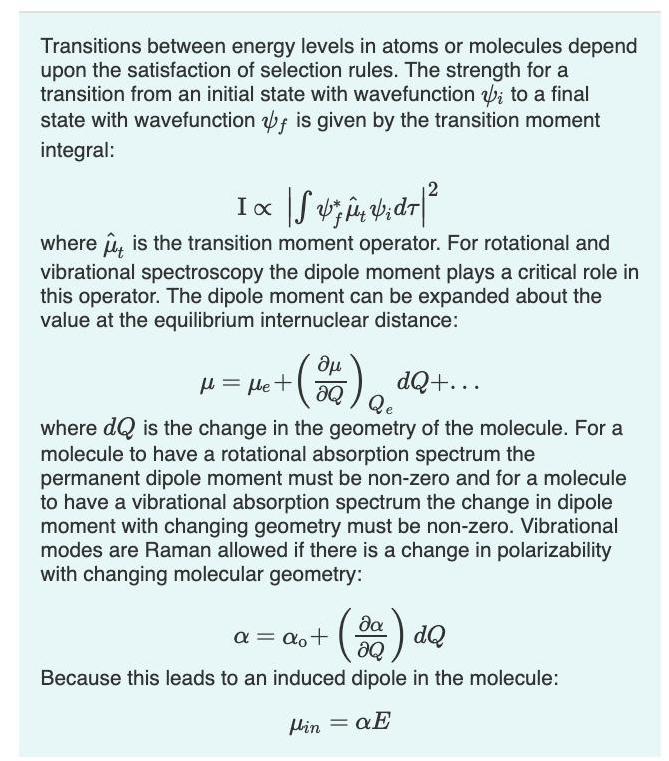
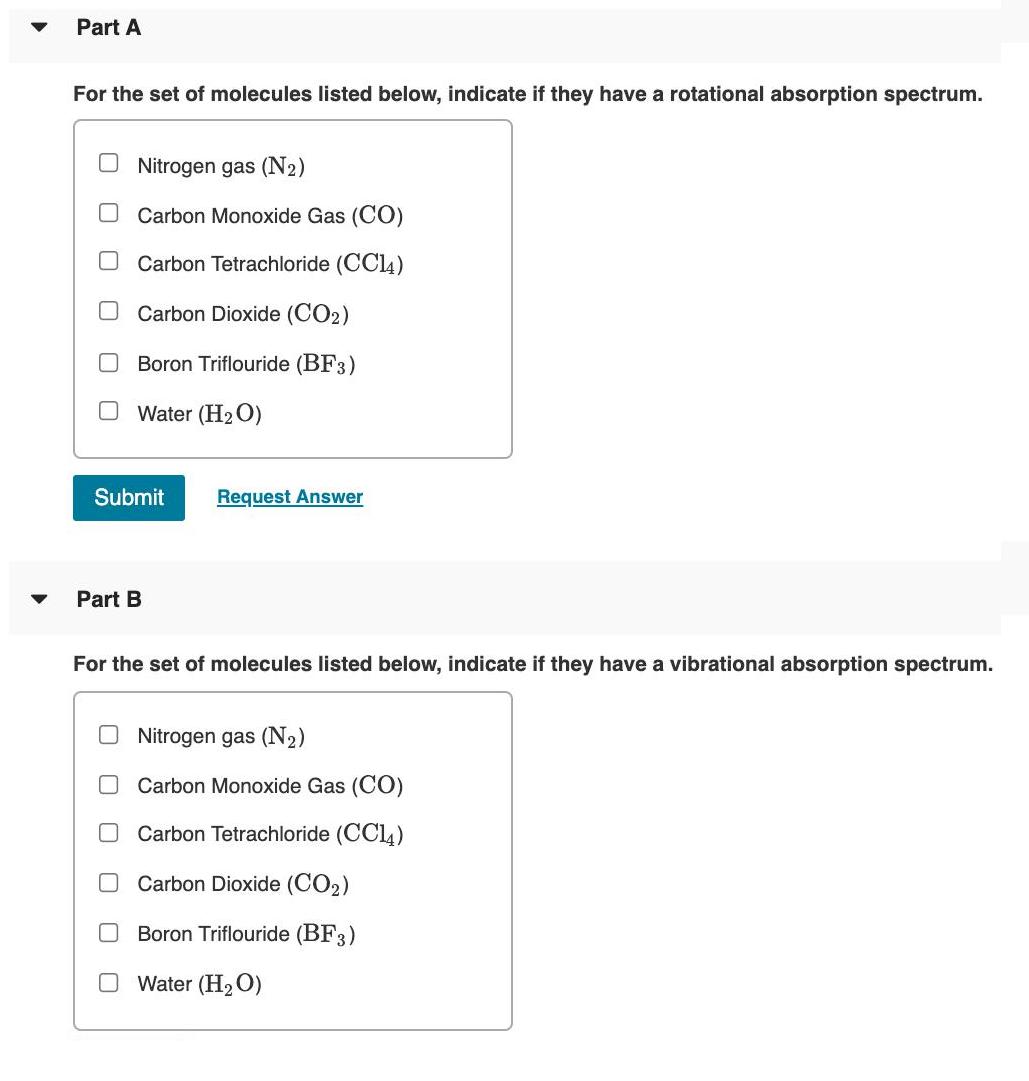
Transitions between energy levels in atoms or molecules depend upon the satisfaction of selection rules. The strength for a transition from an initial state with wavefunction b; to a final state with wavefunction f is given by the transition moment integral: Ix where u, is the transition moment operator. For rotational and vibrational spectroscopy the dipole moment plays a critical role in this operator. The dipole moment can be expanded about the value at the equilibrium internuclear distance: 20 dQ+... Qe where dQ is the change in the geometry of the molecule. For a molecule to have a rotational absorption spectrum the permanent dipole moment must be non-zero and for a molecule to have a vibrational absorption spectrum the change in dipole moment with changing geometry must be non-zero. Vibrational modes are Raman allowed if there is a change in polarizability u = le + with changing molecular geometry: () 40 da a = ao+ aQ Because this leads to an induced dipole in the molecule: %3D Min = aE Part A For the set of molecules listed below, indicate if they have a rotational absorption spectrum. O Nitrogen gas (N2) Carbon Monoxide Gas (CO) Carbon Tetrachloride (CC14) OCarbon Dioxide (CO2) Boron Triflouride (BF3) O Water (H2O) Submit Request Answer Part B For the set of molecules listed below, indicate if they have a vibrational absorption spectrum. O Nitrogen gas (N2) Carbon Monoxide Gas (CO) Carbon Tetrachloride (CCl4) Carbon Dioxide (CO2) Boron Triflouride (BF3) O Water (H2O) O O O O
Step by Step Solution
★★★★★
3.34 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


