Question
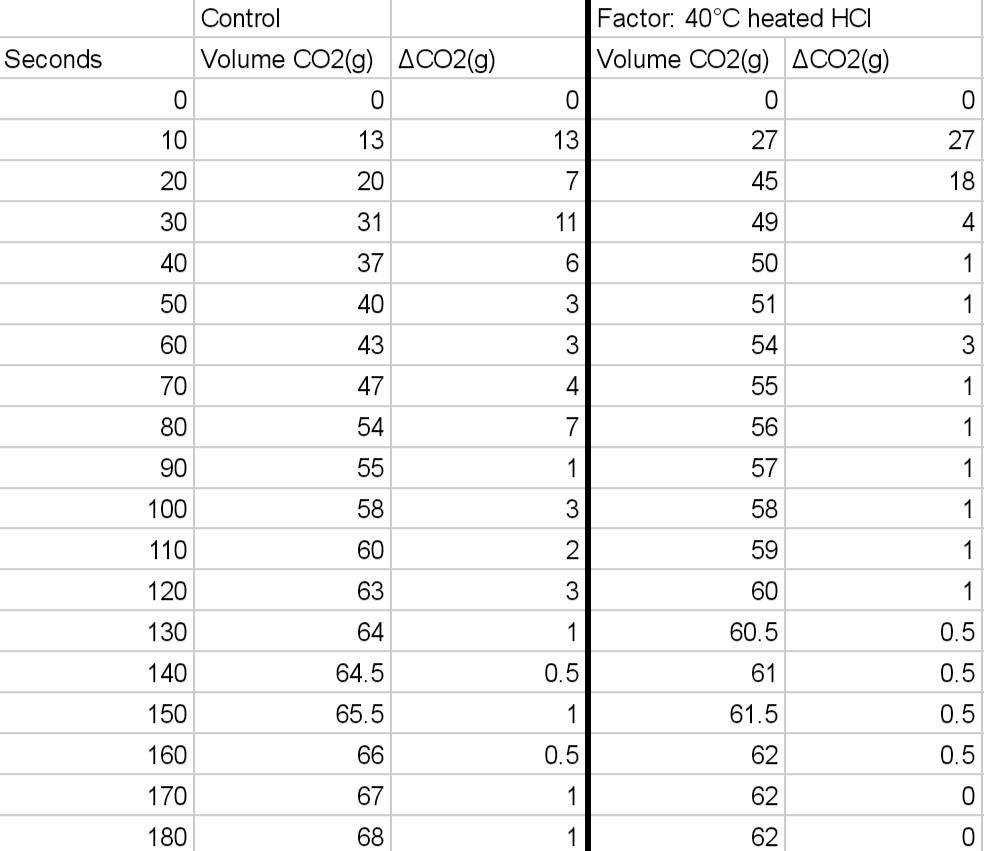
Control Substances: 3.0 g calcium carbonate (chalk) and 0.50 M HCI CaCO3(s) + 2HCl(aq) CaCl2(s) + H2O(aq) + CO2(g) Can you please 2 graphs,
CaCO3(s) + 2HCl(aq) → CaCl2(s) + H2O(aq) + CO2(g)
Can you please 2 graphs, one for the control experiment and one for the variable you chose. The axies should be change in moles of CO2 vs time.
Can you please help me do the following calculations for the sample:
1. The amount of moles of CO2(g) determined for the graphs.
2. The average rate for the reaction of CO2(g), from 30s-70s for each experiment.
3. Instantaneous rate at 45s for each experiment.
Write-up section:
Can you please explain the significance of affecting a reaction's rate by heating the acid HCl. What would be one experimental error that is relevant to the data from the graph/data. Either the main trendline or the outliers in your data collection may be the subject of the inaccuracy.
Conclusion
Can you please explain the findings with a proper scientific conclusion. Since this lab is designed in such a way, percent error is not relevant; instead, please focus the on the hypothesis (if heat factor were to be added, the reaction would speed up) and whether it was accepted or rejected. Can you also please include at least one way to make this experiment's accuracy better.
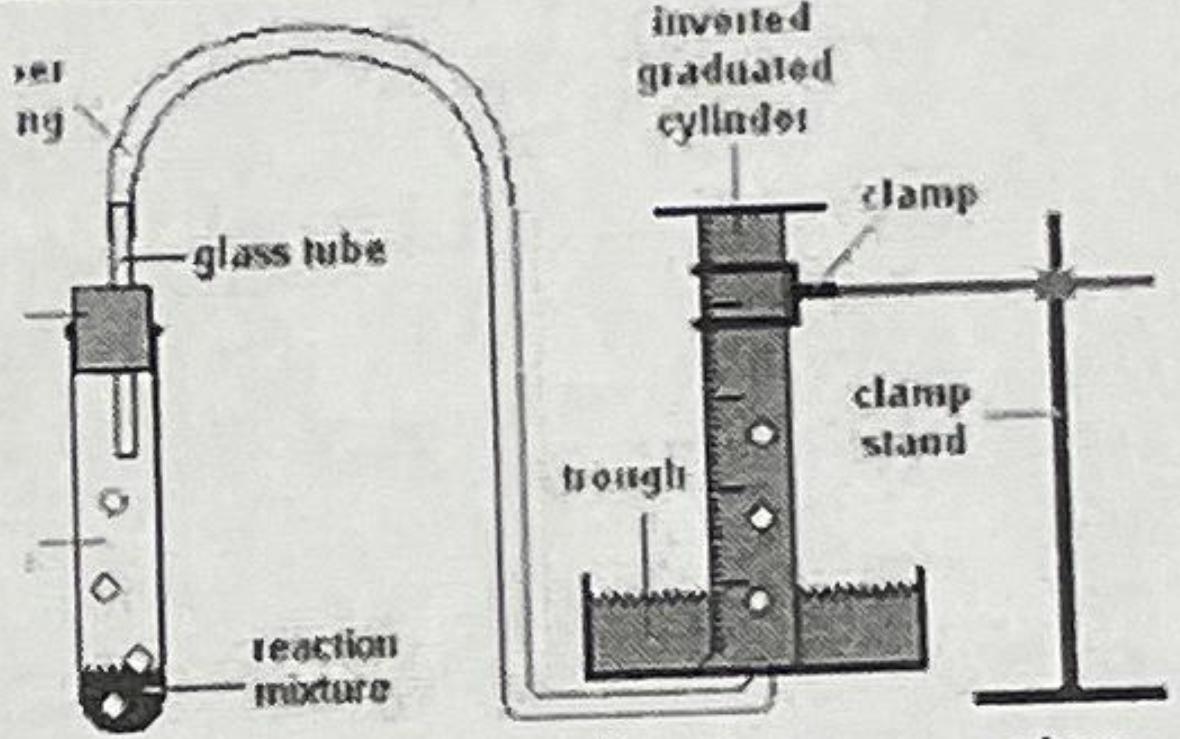
ng glass tube reaction mixture inverted graduated cylinder clamp clamp stand brough
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


