Based on what we learned about the color of organic molecules, do the spectra of the structures labeled 6a - d follow the trend you
Based on what we learned about the color of organic molecules, do the spectra of the structures labeled 6a - d follow the trend you expect? Why?

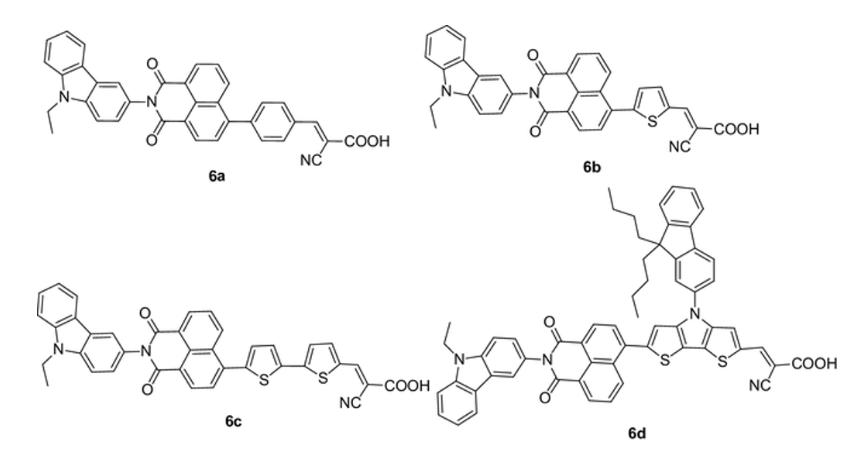
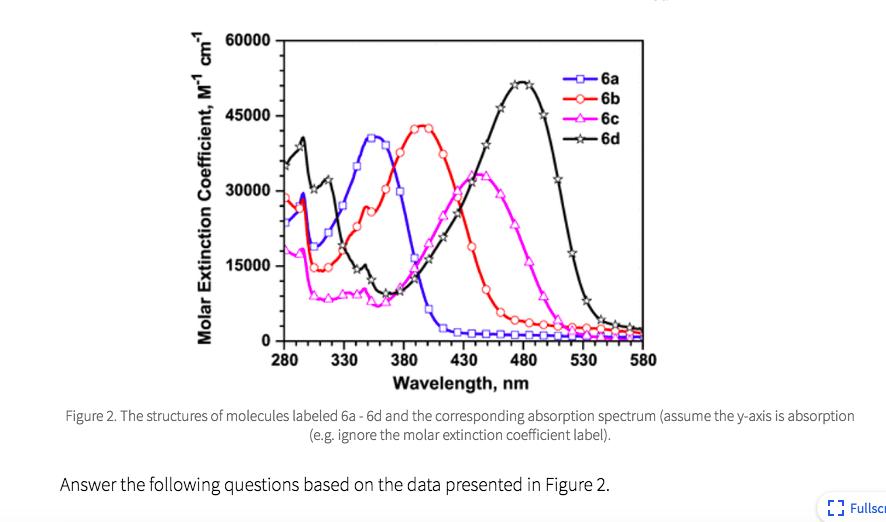
B C D E No. The number of bonds contributing to the largest conjugated part of the molecules, from the shortest to the longest, are 6b, 6a, 6c, and 6d. Therefore, 6b would have the shortest wavelength of maximum absorption and 6d would have the longest wavelength of maximum absorption. However, the experimental spectrum indicates that 6a has the shortest wavelength of the series. Yes. The number of bonds contributing to the largest conjugated part of the molecules, from the shortest to the longest, are 6a, 6b, 6c, and 6d. Therefore, 6a would have the shortest wavelength of maximum absorption and 6d would have the longest wavelength of maximum absorption. The experimental spectrum supports this trend. No. The intensity of the spectrum corresponds to the number of double bonds in the molecule. 6d has the highest number of double bonds and has the spectrum with the highest intensity of the four molecules. However, 6c has the second highest number of double bonds and has the lowest intensity of the four molecules. Therefore, the experimental spectra do not support the current understanding of the molecular properties of organic molecules that contribute to their color. Yes. The intensity of the spectrum corresponds to the number of double bonds in the molecule. 6d has the highest number of double bonds and has the spectrum with the highest intensity of the four molecules. 6b has the lowest number of double bonds and has the lowest intensity of the four molecules. Therefore, the experimental spectra do support the current understanding of the molecular properties of organic molecules that contribute to their color. No. The molecules do not differ in ways that we have discussed should impact the visible spectrum of organic molecules. However, the nitrogen and/or sulfur content increases from 6a to 6d and correlates with the trend in wavelength increases (i.e. more electronegative atoms increases the wavelength). Therefore, the experimental spectra compared to the molecule structure supports a new understanding of the molecular properties of organic molecules that contribute to their color.
Step by Step Solution
3.36 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1
B Yes The number of bonds contributing to the largest conjugated part of the molecules from the shortest to the longest are 6a 6b 6c and 6d Therefore 6a would have the shortest wavelength of maximum a...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


