Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Copyright At the beginning of the nineteenth century, chemists were searching for numerical relation- ships among the elements. From these relationships, they hoped that
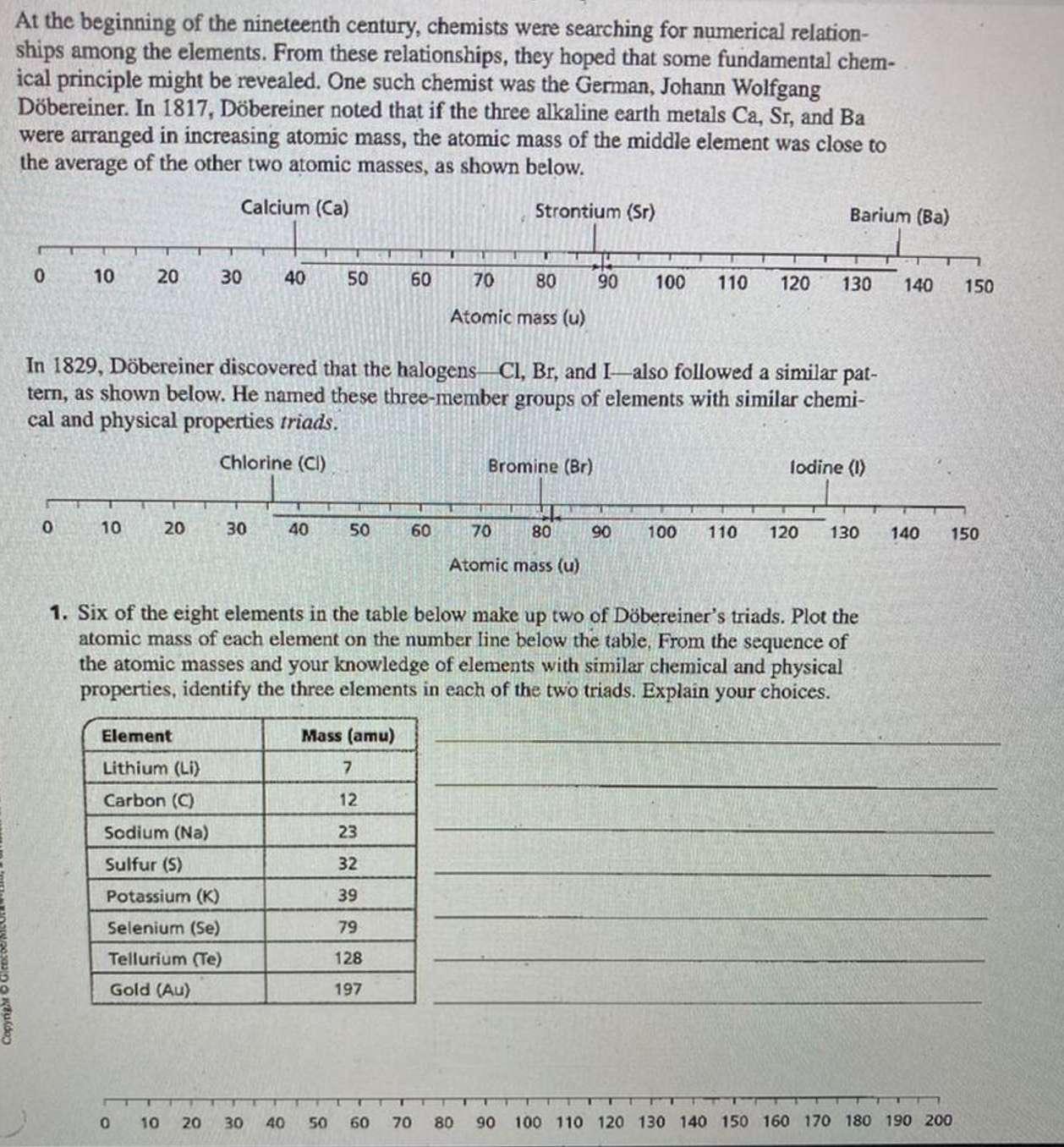
Copyright At the beginning of the nineteenth century, chemists were searching for numerical relation- ships among the elements. From these relationships, they hoped that some fundamental chem- ical principle might be revealed. One such chemist was the German, Johann Wolfgang Dbereiner. In 1817, Dbereiner noted that if the three alkaline earth metals Ca, Sr, and Ba were arranged in increasing atomic mass, the atomic mass of the middle element was close to the average of the other two atomic masses, as shown below. Calcium (Ca) Strontium (Sr) 0 10 0 20 10 20 30 Element Lithium (Li) Carbon (C) Sodium (Na) Sulfur (5) 40 30 Potassium (K) Selenium (Se) Tellurium (Te) Gold (Au) 50 In 1829, Dbereiner discovered that the halogens Cl, Br, and I also followed a similar pat- tern, as shown below. He named these three-member groups of elements with similar chemi- cal and physical properties triads. Chlorine (Cl) 40 0 10 20 30 40 50 60 Mass (amu) 7 12 23 32 39 79 128 197 60 T U 70 80 Atomic mass (u) 50 60 70 U Bromine (Br) 70 80 Atomic mass (u) 80 90 100 110 120 1. Six of the eight elements in the table below make up two of Dbereiner's triads. Plot the atomic mass of each element on the number line below the table, From the sequence of the atomic masses and your knowledge of elements with similar chemical and physical properties, identify the three elements in each of the two triads. Explain your choices. Barium (Ba) 90 130 lodine (1) 100 110 120 130 140 150 140 150 T 90 100 110 120 130 140 150 160 170 180 190 200
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Dobereiners triads represent a classification system for elements that significantly contributed to ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


