Answered step by step
Verified Expert Solution
Question
1 Approved Answer
correct answer is 140 F and 150 Btu. please show work 3. 1.5 lb of a saturated NaOH solution at 70F is mixed with 1.0
correct answer is 140 F and 150 Btu. please show work 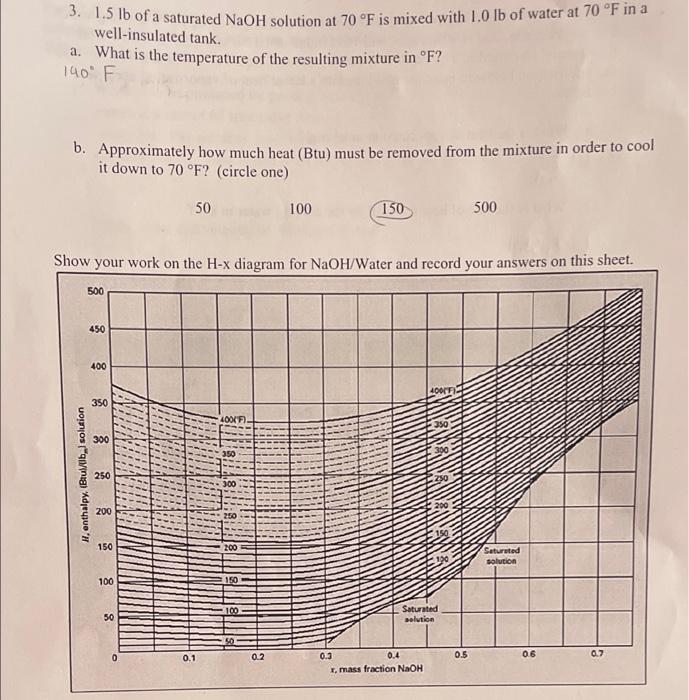
3. 1.5 lb of a saturated NaOH solution at 70F is mixed with 1.0 lb of water at 70F in a a. What is the temperature of the resulting mixture in F? well-insulated tank. 140 b. Approximately how much heat (Btu) must be removed from the mixture in order to cool it down to 70 F? (circle one) 50 100 150 500 Show your work on the H-x diagram for NaOH/Water and record your answers on this sheet. 500 450 400 40019 350 LOKF 350 300 390 H, enthalpy, (Bulb) solution 250 250 300 200 200 150 200 190 Saturated solution 100 150 8 100 50 Saturated solution 5.0 0.1 0.2 0.5 06 0.7 0.3 0.4 I, mass fraction NaOH 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


