Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Correct answers are given, please show work! Answer for a is 36.8 and for b is -50.3 10. The enzyme urease catalyzes the hydrolysis of
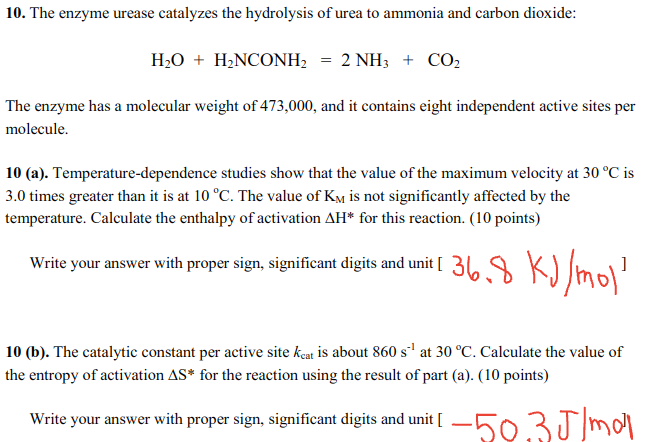
Correct answers are given, please show work! Answer for a is 36.8 and for b is -50.3
10. The enzyme urease catalyzes the hydrolysis of urea to ammonia and carbon dioxide: H2O + H2NCONH2 = 2 NH3 + CO2 The enzyme has a molecular weight of 473,000, and it contains eight independent active sites per molecule. 10 (a). Temperature-dependence studies show that the value of the maximum velocity at 30 C is 3.0 times greater than it is at 10 C. The value of Ky is not significantly affected by the temperature. Calculate the enthalpy of activation AH* for this reaction. (10 points) Write your answer with proper sign, significant digits and unit [ 36.8 kJ/mol ] 10 (b). The catalytic constant per active site keat is about 860 s' at 30 C. Calculate the value of the entropy of activation AS* for the reaction using the result of part (a). (10 points) Write your answer with proper sign, significant digits and unit [ 50,3J/molStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


