Question
Could someone help me figure out how to balance this reactor unit, or at least help me figure out how I would go about it.
Could someone help me figure out how to balance this reactor unit, or at least help me figure out how I would go about it. You are free to make assumptions for the inlet molar flows, I just need help figuring out reactive molar balance.
The reaction is for epsom salt. The balanced equation is H2SO4 + Mg(OH)2 ---> MgSO4 + 2H2O.
Assume Steady-State, open system, you can also assume if you want that 100% of the reactive inlet material is used (even though technically MgSO4 is limiting)
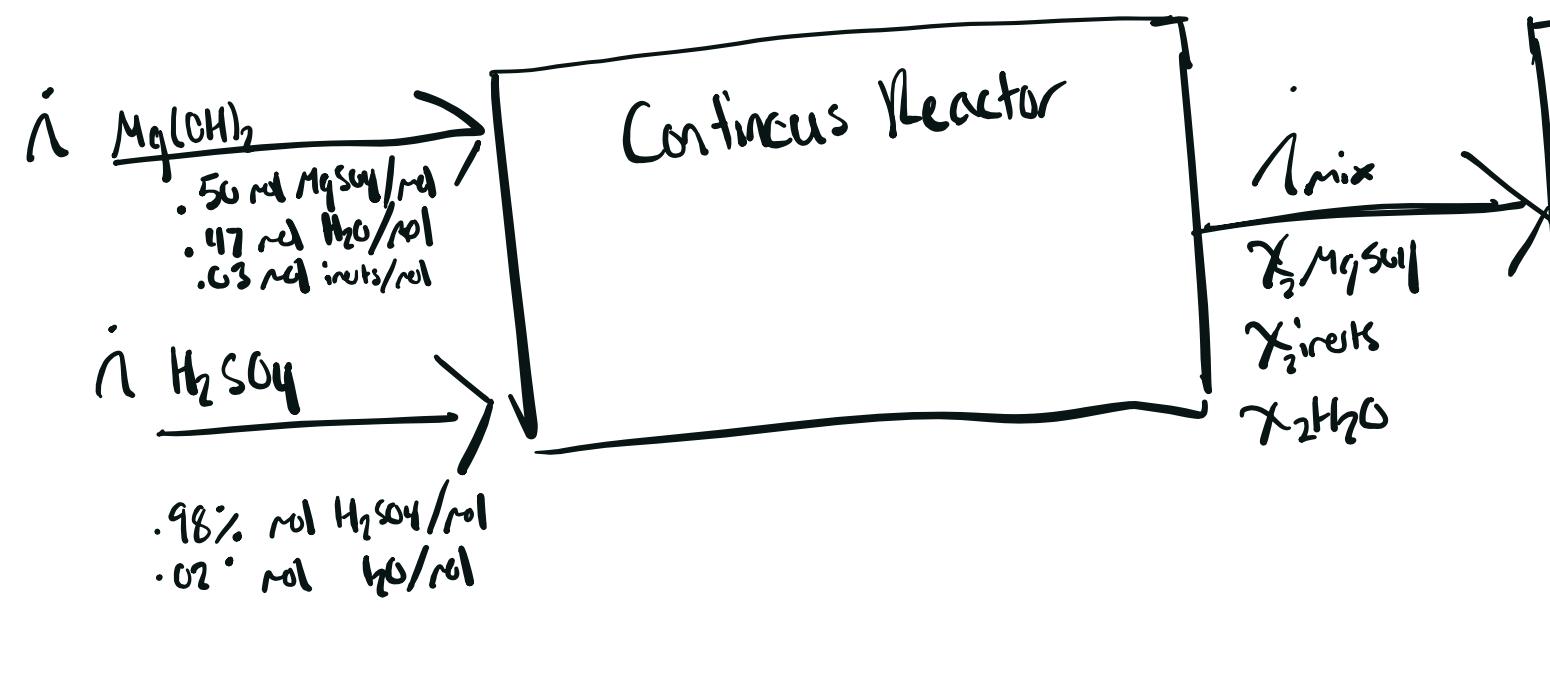
120/04 lov. 20. Mg(OH), Contincus Reactor 50 Mysore 47 ml 1120/10/ Amix . .03 minuts/rel i H soy .98% rol H504/mol trangsay Xirests x2410
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



