Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please show the work for how the following answers were found Using the Steam Table calculate the temperature of a system consisting of water vapor
Please show the work for how the following answers were found
- Using the Steam Table calculate the temperature of a system consisting of water vapor at pressure 5.00 MPa and specific volume 0.08359 m3/kg. Give your answer in ºC.
- 650
- Using the Ideal Gas Law calculate the temperature of a system consisting of water vapor at pressure 5.00 MPa and specific volume 0.08359 m3/kg. The molar mass of water is 0.018 kg/mole. Give your answer in ºC.
- 631
- A closed, rigid tank contains only water vapor and has a dew point of 60°C. The total mass of water in the tank is 10 kg. Using the appropriate Steam Table what is the volume of the tank in m3?
- 77
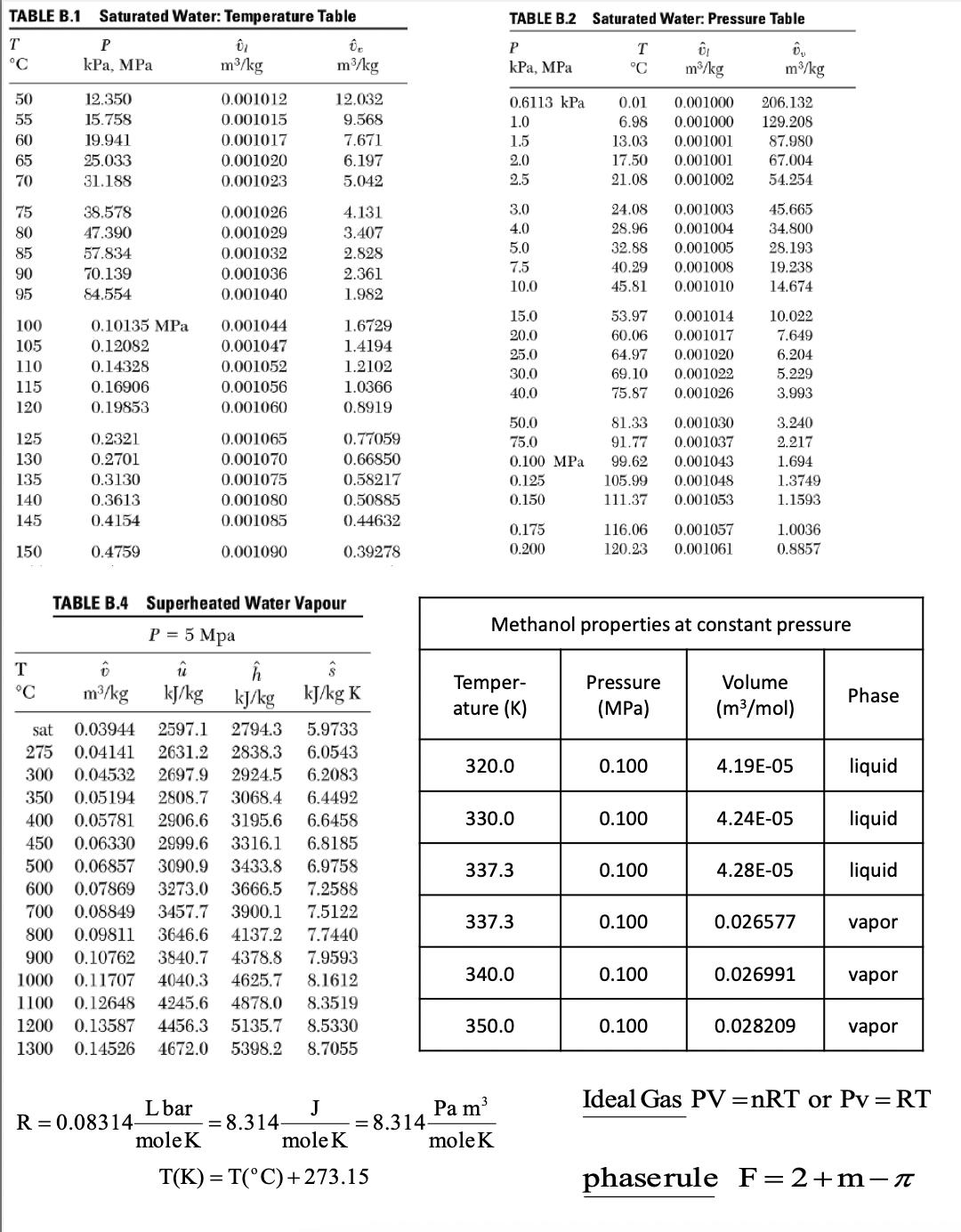
TABLE B.1 Saturated Water: Temperature Table TABLE B.2 Saturated Water: Pressure Table T P C kPa, MPa m/kg P T m/kg kPa, MPa C m/kg m/kg 50 12.350 0.001012 12.032 0.6113 kPa 55 15.758 0.001015 9.568 1.0 0.01 0.001000 206.132 6.98 0.001000 129.208 60 19.941 0.001017 7.671 1.5 13.03 0.001001 87.980 65 25.033 0.001020 6.197 2.0 17.50 0.001001 67.004 70 31.188 0.001023 5.042 2.5 21.08 0.001002 54.254 75 38.578 0.001026 4.131 3.0 24.08 0.001003 45.665 80 47.390 0.001029 3.407 4.0 28.96 0.001004 34.800 85 57.834 0.001032 2.828 5.0 32.88 0.001005 28.193 7.5 40.29 0.001008 19.238 90 70.139 0.001036 2.361 10.0 45.81 0.001010 14.674 95 84.554 0.001040 1.982 15.0 53.97 0.001014 10.022 100 0.10135 MPa 0.001044 1.6729 20.0 60.06 0.001017 7.649 105 0.12082 0.001047 1.4194 25.0 64.97 0.001020 6.204 110 0.14328 0.001052 1.2102 30.0 69.10 0.001022 5.229 115 0.16906 0.001056 1.0366 40.0 75.87 0.001026 3.993 120 0.19853 0.001060 0.8919 50.0 81.33 0.001030 3.240 125 0.2321 0.001065 0.77059 75.0 91.77 0.001037 2.217 130 0.2701 0.001070 0.66850 0.100 MPa 99.62 0.001043 1.694 135 0.3130 0.001075 0.58217 0.125 105.99 0.001048 1.3749 140 0.3613 0.001080 0.50885 0.150 111.37 0.001053 1.1593 145 0.4154 0.001085 0.44632 0.175 150 0.4759 0.001090 0.39278 0.200 116.06 0.001057 120.23 0.001061 1.0036 0.8857 TABLE B.4 Superheated Water Vapour P = 5 Mpa Methanol properties at constant pressure T C m/kg kJ/kg kJ/kg kJ/kg K Temper- Pressure ature (K) (MPa) Volume (m/mol) Phase sat 0.03944 2597.1 2794.3 5.9733 275 0.04141 2631.2 2838.3 6.0543 300 0.04532 350 0.05194 400 2697.9 2924.5 6.2083 2808.7 3068.4 6.4492 0.05781 2906.6 3195.6 450 0.06330 2999.6 3316.1 500 0.06857 3090.9 3433.8 6.9758 600 0.07869 3273.0 3666.5 7.2588 700 0.08849 3457.7 3900.1 7.5122 800 0.09811 3646.6 4137.2 7.7440 900 0.10762 3840.7 4378.8 7.9593 1000 0.11707 4625.7 4040.3 8.1612 1100 0.12648 4245.6 4878.0 8.3519 1200 0.13587 4456.3 5135.7 8.5330 1300 0.14526 4672.0 5398.2 8.7055 320.0 0.100 4.19E-05 liquid 6.6458 330.0 0.100 4.24E-05 liquid 6.8185 337.3 0.100 4.28E-05 liquid 337.3 0.100 0.026577 vapor 340.0 0.100 0.026991 vapor 350.0 0.100 0.028209 vapor R 0.08314- Lbar = 8.314- mole K J Pa m Ideal Gas PV = nRT or Pv = RT =8.314- mole K mole K T(K) T(C)+273.15 phase rule F=2+m-
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


