Answered step by step
Verified Expert Solution
Question
1 Approved Answer
could you please answer all of them. I really appreciate. thank you so muchh Give IUPAC names for the following structures. (If appropriate, specify relative
could you please answer all of them. I really appreciate. thank you so muchh 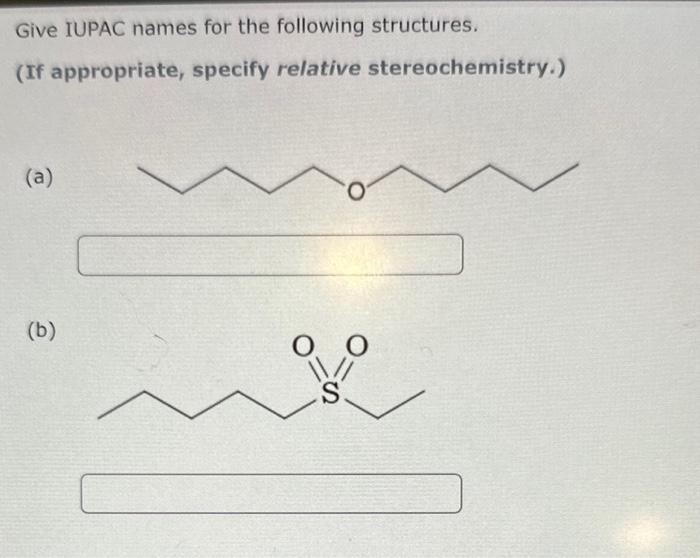
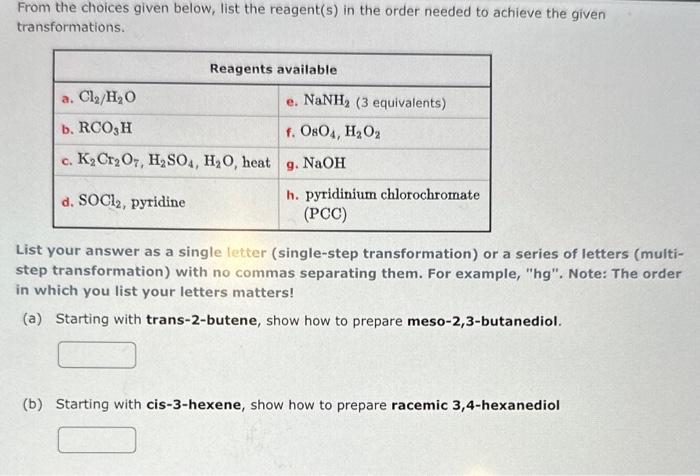
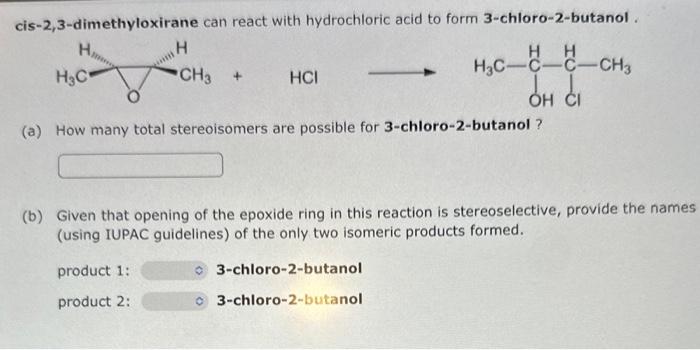
Give IUPAC names for the following structures. (If appropriate, specify relative stereochemistry.) (a) (b) From the choices given below, list the reagent(s) in the order needed to achieve the given transformations. List your answer as a single letter (single-step transformation) or a series of letters (multistep transformation) with no commas separating them. For example, "hg". Note: The order in which you list your letters matters! (a) Starting with trans-2-butene, show how to prepare meso-2,3-butanediol. (b) Starting with cis-3-hexene, show how to prepare racemic 3,4-hexanediol cis-2,3-dimethyloxirane can react with hydrochloric acid to form 3-chloro-2-butanol . (a) How many total stereoisomers are possible for 3-chloro-2-butanol? (b) Given that opening of the epoxide ring in this reaction is stereoselective, provide the names (using IUPAC guidelines) of the only two isomeric products formed. product 1: 3-chloro-2-butanol product 2: 3-chloro-2-butanol 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


