Answered step by step
Verified Expert Solution
Question
1 Approved Answer
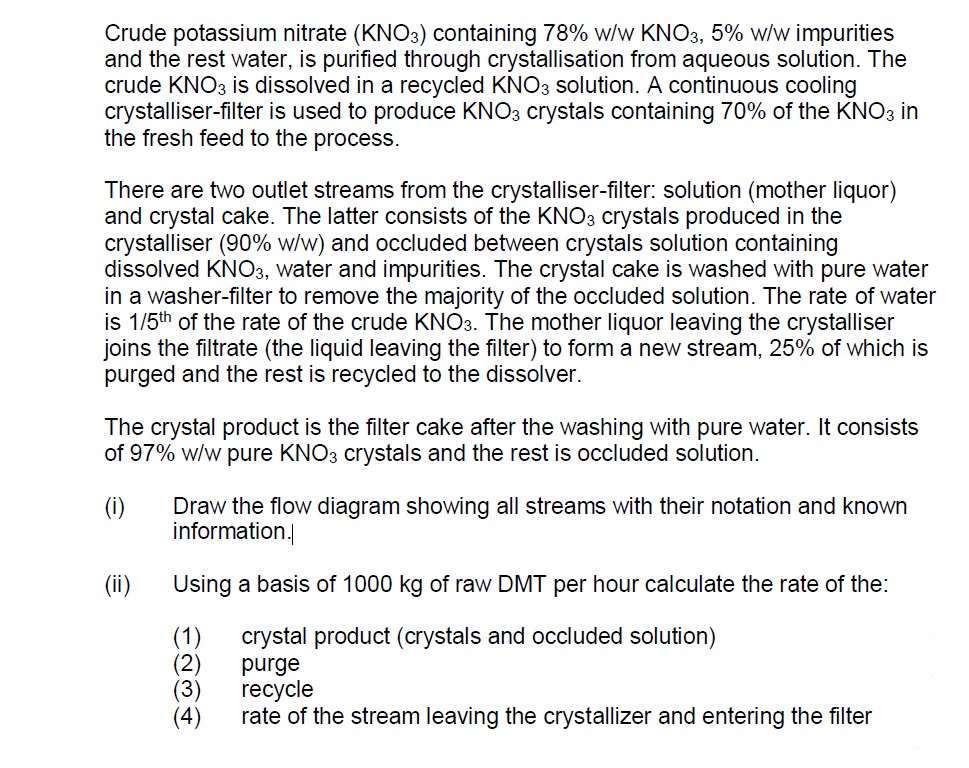
Crude potassium nitrate ( KNO 3 ) containing 7 8 % w / w KNO 3 , 5 % w / w impurities and the
Crude potassium nitrate KNO containing ww KNO ww impurities and the rest water, is purified through crystallisation from aqueous solution. The crude KNO is dissolved in a recycled KNO solution. A continuous cooling crystalliserfilter is used to produce KNO crystals containing of the KNO in the fresh feed to the process.
There are two outlet streams from the crystalliserfilter: solution mother liquor and crystal cake. The latter consists of the KNO crystals produced in the crystalliser ww and occluded between crystals solution containing dissolved KNO water and impurities. The crystal cake is washed with pure water in a washerfilter to remove the majority of the occluded solution. The rate of water is th of the rate of the crude KNO The mother liquor leaving the crystalliser joins the filtrate the liquid leaving the filter to form a new stream, of which is purged and the rest is recycled to the dissolver.
The crystal product is the filter cake after the washing with pure water. It consists of ww pure KNO crystals and the rest is occluded solution
i Draw the flow diagram showing all streams with their notation and known information
Using a basis of kg of raw DMT per hour calculate the rate of the:
crystal product crystals and occluded solution
purge
recycleand the rest water, is purified through crystallisation from aqueous solution. The
crude is dissolved in a recycled solution. A continuous cooling
crystalliserfilter is used to produce crystals containing of the in
the fresh feed to the process.
There are two outlet streams from the crystalliserfilter: solution mother liquor
and crystal cake. The latter consists of the crystals produced in the
crystalliser and occluded between crystals solution containing
dissolved water and impurities. The crystal cake is washed with pure water
in a washerfilter to remove the majority of the occluded solution. The rate of water
is of the rate of the crude The mother liquor leaving the crystalliser
joins the filtrate the liquid leaving the filter to form a new stream, of which is
purged and the rest is recycled to the dissolver.
The crystal product is the filter cake after the washing with pure water. It consists
of pure crystals and the rest is occluded solution.
i Draw the flow diagram showing all streams with their notation and known
information.
ii Using a basis of of raw DMT per hour calculate the rate of the:
crystal product crystals and occluded solution
purge
recycle
rate of the stream leaving the crystallizer and entering the filter
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


