Question
Crystallization of NaOH from 10%-mass NaOH solution was carried out as follows: 10% NaOH solution (F1, temperature 60F) was mixed in Unit-I with the crystallization
Crystallization of NaOH from 10%-mass NaOH solution was carried out as follows: 10% NaOH solution (F1, temperature 60F) was mixed in Unit-I with the crystallization induction solution of Unit-III (mother liquor, F2). The mixture of these two streams (F3) is fed to the evaporation unit (Unit II). Evaporation was carried out at a temperature of 200F. Some of the water in the vaporized solution (F4) and the solution (F5) had a 70%-mass concentration of NaOH. After evaporation, the solution (F5) was successively treated in Unit-III consisting of cooling, crystallization, and separation of the crystals from the mother liquor. NaOH crystals were taken as product (F6, top product Unit-III) and considered pure. Saturated solution of NaOH temperature 80F (F2) as the bottom product of Unit-III was mixed back into the fresh feed in Unit-I. Flow data at a pressure of 1 atm, obtained from the Enthalpy-Composition diagram. Question : a. Complete the above data through mass and energy balance calculations b. Write an analysis of the degrees of freedom of the mass balance involving the energy balance of the problem above (Unit-I, Unit-II, Unit-III, Overall, and Process)
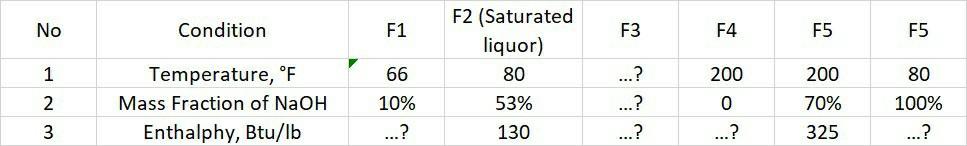
No Condition F1 F3 F4 F5 F5 F2 (Saturated liquor) 80 53% 66 WNA Z ...? 200 200 80 Temperature, F Mass Fraction of NaOH Enthalphy, Btu/lb 100% 10% ...? 0 ...? 70% 325 130
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


