CuSO4.xH2O
mass of empty beaker = 29.516g
mass of hydrate = 0.598g
Time in oven = 20 min
Cooling time = 10 min
Mass of beaker with anhydrous salt = 29.939 g
mass of anhydrous salt = 0.423g
mass of water evaporated = 0.175g
moles of water = 9.71x10^-3 mol
moles of anhydrous salt = 2.65x10^-3 mol
hydration number ~ 4
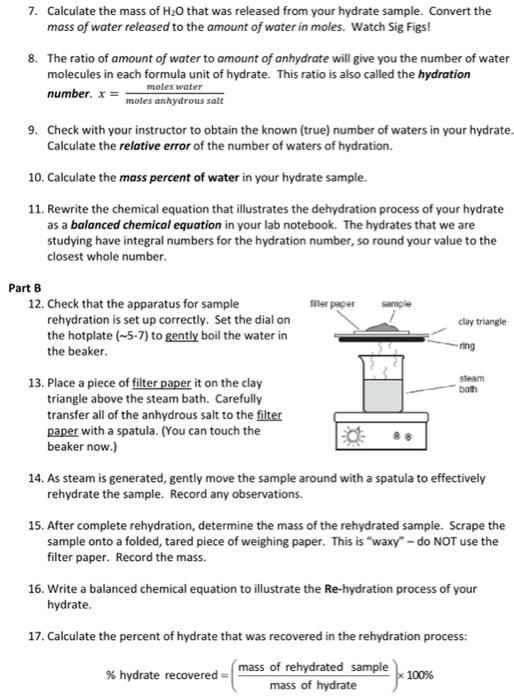
1. You will find a desiccator with a labeled beaker inside cooling. Record the beaker label. Tare the balance. Open the desiccator and move the beaker to the balance using tongs. Record the mass of the empty beaker to the appropriate number of significant figures. 2. Obtain a sample bottle - what are your observations? Record the chemical formula of the hydrate from the bottle label. Tare the balance with the empty beaker still in place. Add approximately 0.40.6_g of the hydrate to the beaker using a spatula. Recoro the mass of the hydrate sample. Use the spatula to break up any large chunks of sample. Note any significant observations. Return the beaker to the desiccator with tong. 3. Take your desiccator/sample to the oven. Using tongs, place your hydrate sample in the oven at 110C to dry for 2025min. After drying, the samples will be allowed to cool for a few minutes (10-15) in a desiccator. Record your observations. 4. While you are waiting for your hydrate sample to dry, you will need to complete the following: - Write a chemical equation to illustrate the dehydration process of your hydrate; - Calculate the molar mass (also called formula mass) of water; - Calculate the molar mass of your anhydrate; - Turn on the steam bath so that the water is at a gentle boil (Shown in Part B). 5. After your dehydrated sample has cooled, tare the balance. Using tongs, move the anhydrous sample/beaker to the balance and record the mass. 6. Calculate the mass of the anhydrate sample in the beaker to the correct number of significant figures. Convert the mass of the anhydrate to the amount of anhydrate in moles. Watch Sig Figs! 7. Calculate the mass of H2O that was released from your hydrate sample. Convert the mass of water released to the amount of water in moles. Watch Sig Figs! 8. The ratio of amount of water to amount of anhydrate will give you the number of water molecules in each formula unit of hydrate. This ratio is also called the hydration number. x=molesanhydroussaltmoleswater 9. Check with your instructor to obtain the known (true) number of waters in your hydrate. Calculate the relative error of the number of waters of hydration. 10. Calculate the mass percent of water in your hydrate sample. 11. Rewrite the chemical equation that illustrates the dehydration process of your hydrate as a balanced chemical equation in your lab notebook. The hydrates that we are studying have integral numbers for the hydration number, so round your value to the closest whole number. 13. Place a piece of filter paper it on the clay triangle above the steam bath. Carefully transfer all of the anhydrous salt to the filter paper with a spatula. (You can touch the beaker now.) 14. As steam is generated, gently move the sample around with a spatula to effectively rehydrate the sample. Record any observations. 15. After complete rehydration, determine the mass of the rehydrated sample. Scrape the sample onto a folded, tared piece of weighing paper. This is "waxy" - do NOT use the filter paper. Record the mass. 16. Write a balanced chemical equation to illustrate the Re-hydration process of your hydrate. 17. Calculate the percent of hydrate that was recovered in the rehydration process: %hydraterecovered=(massofhydratemassofrehydratedsample)100% Questions 1. It IS possible to overheat the hydrate by using a temperature that not only drives off the water but also chars the anhydrous salt. Would this overheating increase, decrease, or result in no change of the hydration number? Explain why. 2. If we had more time, the sample would have been heated and cooled twice to make sure all of the water was evaporated. What observation suggested that the water was removed with only one heating cycle? 3. What error (if any) would occur if you touched the beaker before measuring the mass of the anhydrous salt? What error (if any) would occur if you touched the beaker after measuring the mass of the anhydrous salt? 4. In the rehydration process, were you able to recover 100% of the original hydrate sample? Why or why not? 5. In Step \#15 you were told to mass your sample on weighing paper not on the filter paper. Explain why? If you did use the filter paper, what errors would occur? 6. Calculate the molar mass of CuSO45H2O. Determine the mass percent of water (H2O) and the mass percent of copper (Cu) in this hydrate