Answered step by step
Verified Expert Solution
Question
1 Approved Answer
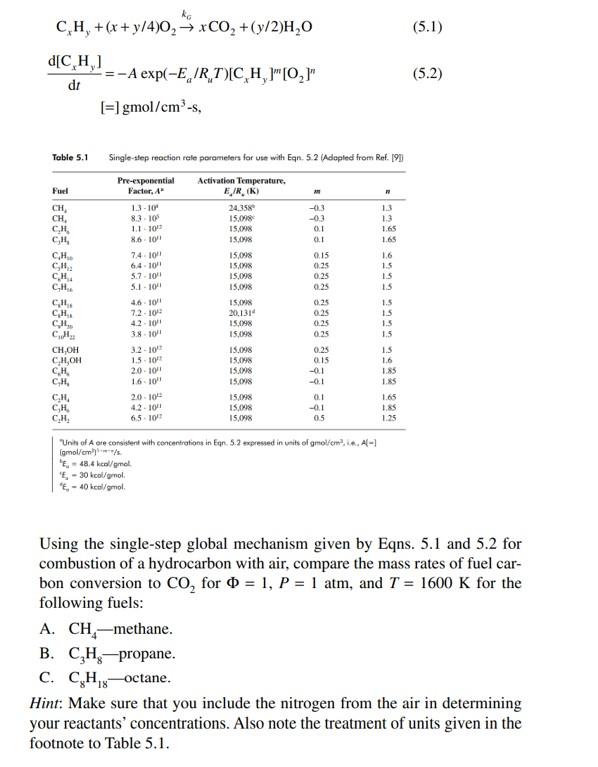
CxHy+(x+y/4)O2kxCO2+(y/2)H2Odtd[CxHy]=Aexp(Ea/RuT)[CxHy]m[O2]n[=]gmol/cm3s, Toble 5.1 Single-slep reoctian rale peramelers for use with Eqn. 5.2 (Adepled from Ref. [9]) Using the single-step global mechanism given by Eqns. 5.1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


