Answered step by step
Verified Expert Solution
Question
1 Approved Answer
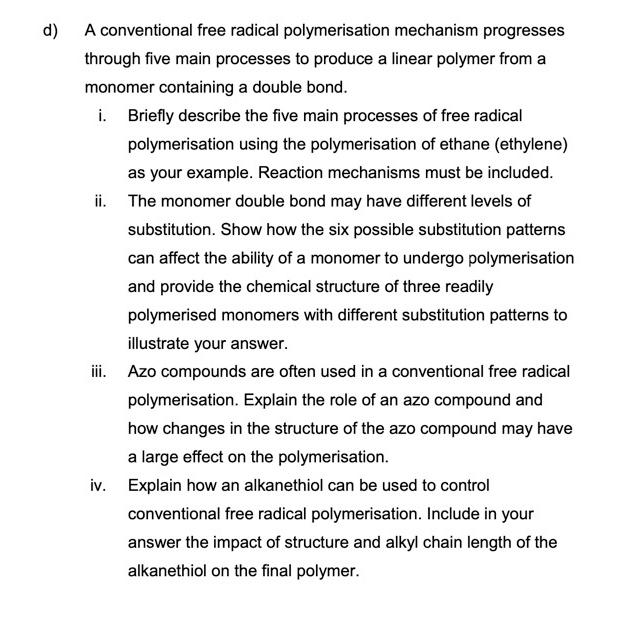
d ) A conventional free radical polymerisation mechanism progresses through five main processes to produce a linear polymer from a monomer containing a double bond.
d A conventional free radical polymerisation mechanism progresses through five main processes to produce a linear polymer from a monomer containing a double bond.
i Briefly describe the five main processes of free radical polymerisation using the polymerisation of ethane ethylene as your example. Reaction mechanisms must be included.
ii The monomer double bond may have different levels of substitution. Show how the six possible substitution patterns can affect the ability of a monomer to undergo polymerisation and provide the chemical structure of three readily polymerised monomers with different substitution patterns to illustrate your answer.
iii. Azo compounds are often used in a conventional free radical polymerisation. Explain the role of an azo compound and how changes in the structure of the azo compound may have a large effect on the polymerisation.
iv Explain how an alkanethiol can be used to control conventional free radical polymerisation. Include in your answer the impact of structure and alkyl chain length of the alkanethiol on the final polymer.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


