Answered step by step
Verified Expert Solution
Question
1 Approved Answer
D. Natural gas (approximated as propyne (C3H4)) is used to provide the energy in the boiler of a steam power plant to add energy
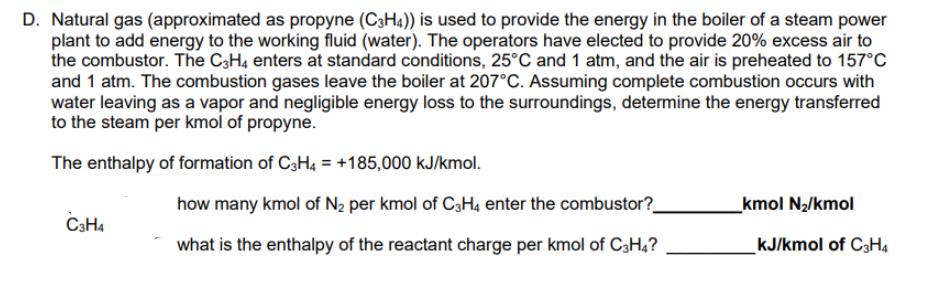
D. Natural gas (approximated as propyne (C3H4)) is used to provide the energy in the boiler of a steam power plant to add energy to the working fluid (water). The operators have elected to provide 20% excess air to the combustor. The C3H4 enters at standard conditions, 25C and 1 atm, and the air is preheated to 157C and 1 atm. The combustion gases leave the boiler at 207C. Assuming complete combustion occurs with water leaving as a vapor and negligible energy loss to the surroundings, determine the energy transferred to the steam per kmol of propyne. The enthalpy of formation of C3H4 = +185,000 kJ/kmol. C3H4 how many kmol of N per kmol of C3H4 enter the combustor?_ what is the enthalpy of the reactant charge per kmol of C3H4? _kmol N/kmol kJ/kmol of C3H4
Step by Step Solution
★★★★★
3.36 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
In order to determine the energy transferred ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


