Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1 Water enters a continuous flow gas calorimeter at 65 F and leaves at 95 F. During a five minute run 0.253 ft 3
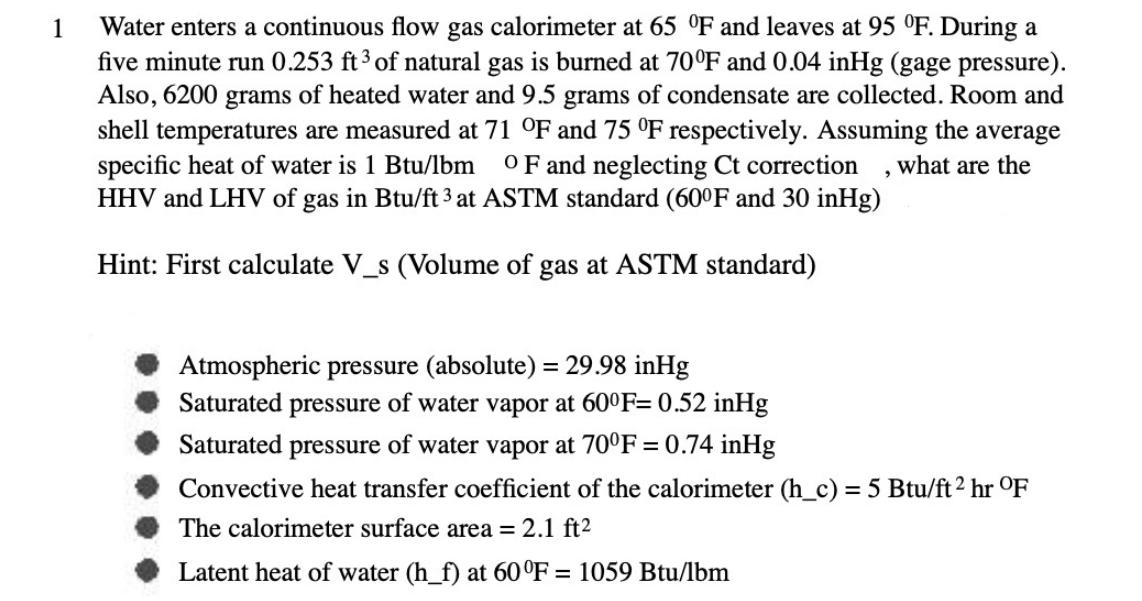
1 Water enters a continuous flow gas calorimeter at 65 F and leaves at 95 F. During a five minute run 0.253 ft 3 of natural gas is burned at 70F and 0.04 inHg (gage pressure). Also, 6200 grams of heated water and 9.5 grams of condensate are collected. Room and shell temperatures are measured at 71 F and 75 F respectively. Assuming the average specific heat of water is 1 Btu/lbm OF and neglecting Ct correction what are the HHV and LHV of gas in Btu/ft 3 at ASTM standard (60F and 30 inHg) Hint: First calculate V_s (Volume of gas at ASTM standard) 9 Atmospheric pressure (absolute) = 29.98 inHg Saturated pressure of water vapor at 600F= 0.52 inHg Saturated pressure of water vapor at 70F= 0.74 inHg Convective heat transfer coefficient of the calorimeter (h_c) = 5 Btu/ft hr OF The calorimeter surface area = 2.1 ft Latent heat of water (h_f) at 60F = 1059 Btu/lbm
Step by Step Solution
★★★★★
3.39 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
HHV Q total H vap LHV Q total H cond Q total m gas H comb H comb ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


