Question
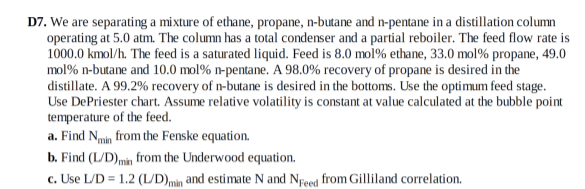
D7. We are separating a mixture of ethane, propane, n-butane and n-pentane in a distillation column operating at 5.0atm . The column has a total
D7. We are separating a mixture of ethane, propane, n-butane and n-pentane in a distillation column operating at
5.0atm. The column has a total condenser and a partial reboiler. The feed flow rate is
1000.0kmo(l)/(h). The feed is a saturated liquid. Feed is
8.0mol%ethane,
33.0mol%propane, 49.0
mol%n-butane and
10.0mol%n-pentane. A
98.0%recovery of propane is desired in the distillate. A 99.2% recovery of n-butane is desired in the bottoms. Use the optimum feed stage. Use DePriester chart. Assume relative volatility is constant at value calculated at the bubble point temperature of the feed.\ a. Find
N_(min)from the Fenske equation.\ b. Find
((L)/(D))_(min)from the Underwood equation.\ c. Use
(L)/(D)=1.2((L)/(D))_(min)and estimate
Nand
N_(Feed )from Gilliland correlation.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


