Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For one of the binary systems listed in Table 13.10, based on Eq. (13.19) and the Wil- son equation, make the following calculations: (a)
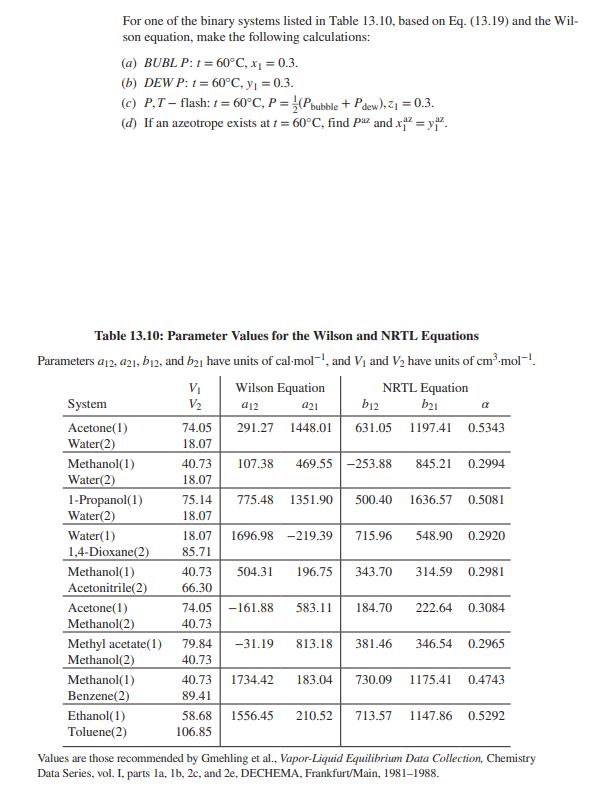
For one of the binary systems listed in Table 13.10, based on Eq. (13.19) and the Wil- son equation, make the following calculations: (a) BUBLP: 1 = 60C, x = 0.3. (b) DEW P: 1= 60C, y = 0.3. (c) P,T-flash: t=60C, P = (Pbubble + Pdew), Z = 0.3. (d) If an azeotrope exists at t=60C, find Paz and x=yiz. Table 13.10: Parameter Values for the Wilson and NRTL Equations Parameters a12, 421, b12, and b21 have units of cal-moll, and V and V2 have units of cm.mol-1. Wilson Equation System V V2 a12 a21 b12 Acetone(1) 74.05 291.27 1448.01 631.05 NRTL Equation b21 1197.41 0.5343 Water(2) 18.07 Methanol(1) 40.73 107.38 469.55 -253.88 845.21 0.2994 Water(2) 18.07 1-Propanol(1) Water(2) 75.14 775.48 1351.90 500.40 18.07 1636.57 0.5081 Water(1) 1,4-Dioxane(2) 18.07 1696.98 -219.39 85.71 715.96 548.90 0.2920 Methanol(1) Acetonitrile(2) 40.73 504.31 196.75 66.30 343.70 314.59 0.2981 Acetone(1) Methanol(2) 74.05 -161.88 583.11 184.70 40.73 222.64 0.3084 Methyl acetate(1) Methanol(2) 79.84 -31.19 813.18 40.73 381.46 346.54 0.2965 Methanol(1) Benzene(2) 40.73 1734.42 183.04 730.09 89.41 1175.41 0.4743 Ethanol(1) Toluene(2) 58.68 1556.45 210.52 106.85 713.57 1147.86 0.5292 Values are those recommended by Gmehling et al., Vapor-Liquid Equilibrium Data Collection, Chemistry Data Series, vol. I, parts la, lb, 2c, and 2e, DECHEMA, Frankfurt/Main, 1981-1988.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


