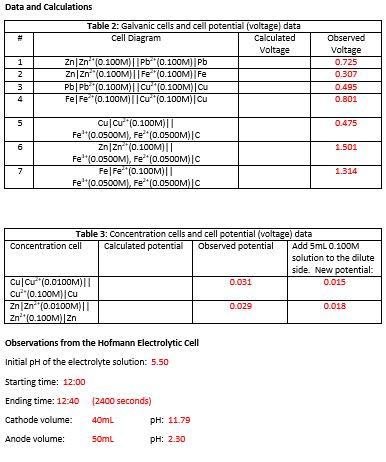


Data and Calculations #1: Table 2: Galvanic cells and cell potential (voltage data Cell Diagram Calculated Voltage ZnZn* 0.100M) || Pb (0.100M) Pb ZnZn (0.100M)||Fe (0.100M)|Fe PbPb (0.100M)||Cu (0.100M) Ou Fe|Fe (0.100M)||CU 10.100M) Cu 1 2 3 4 Observed Voltage 0.725 0.307 0.495 0.801 Nm 5 5 0.475 6 1.501 Cucu (0.100M) Fe(0.0500M), Fe*(0.0SOOM)C ZnZn (0.100M) || Fel:(0.0500M), Fe*(0.05OOM) Fe Fe (0.100M)|| Fe' (0.0500M), Fe**(0.05OOM)C 7 1.314 Table 3: Concentration cells and cell potential voltage) data Concentration cell Calculated potential Observed potential Add 5mL 0.100M solution to the dilute side. New potential: Cu Cu (0.0100M) 0.031 0.015 c"{0.10CM|| Cu ZnZn (0.0100M/II 0.029 0.018 Zn (0.100M Zn Observations from the Hofmann Electrolytic Cell Initial pH of the electrolyte solution: 5.50 Starting time: 12:00 Ending time: 12:40 (2400 seconds) Cathode volume: 40mL PH: 11.79 Anode volume: Soml PH: 2.30 Questions 1. Using the currents calculated from H* production at the anode and then OH production at the cathode, calculate the average current of the power supply that was connected to the electrolytic cell. 2. Give balanced chemical equations for all of the galvanic cells in Table 12.2. 1. 2. 3. 4. 5. 6. 7. 3. This experiment required that you change solutions between electrochemical cells even if they use the same reagents; otherwise, potentials might become less and less accurate. Why was this the case? 4. Use the chemical equation for the redox reaction and the Nernst equation to answer the following questions: a. Show why a battery using the cell CdCd+(1.5M)||Ni2+(1.5M)|Ni would eventually die (its potential would get too low to power a device) even though neither the Cd2+ nor the Ni2+ is completely gone. b. Would you expect a battery using the cell Zn/Zn*||Fe, Fe2+|Pt to last longer than the cell in part a? Support your answer. 5. Different batteries have different driving forces behind why they work. Address each type of battery in the following situations. a. Compare the calculated potentials of your two concentration cells from Table 12.3 before adding additional metal ions. What makes these cells have a positive electric potential in a concentration cell? Does the comparison make sense? Explain your rationale. b. What happened to the electric potential of your concentration cell after adding more metal ions to the dilute side? Is this expected? Explain what happened using Le Chtelier's principle to connect the equilibrium with the electric potential values. c. In the cells in Table 12.2 the metal ions of both the anode and cathode were the same concentration. Explain what makes these cells produce a positive electric potential









