Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Data and Calculations Partner(s) Part 1: Determining the calorimeter constant: Trial Trial g 60 60 g g Data (a) Mass of empty Styrofoam cups (b)
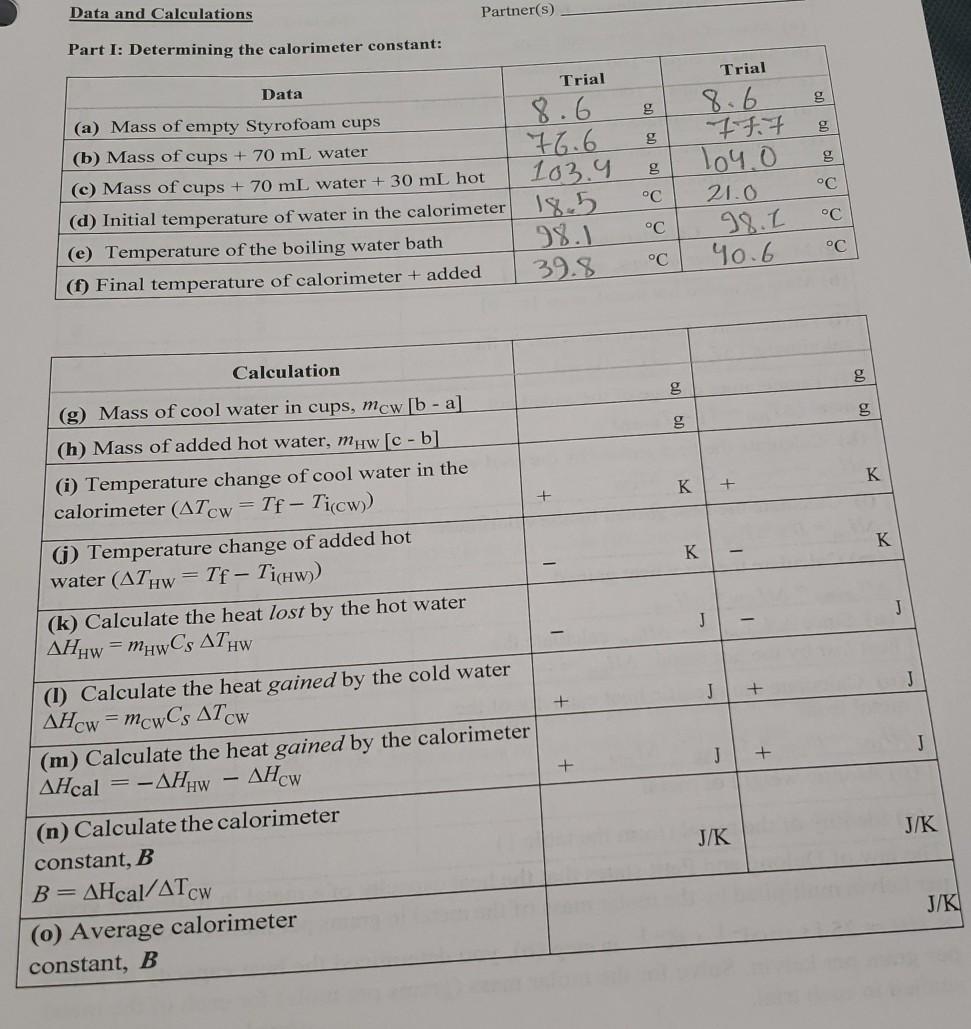
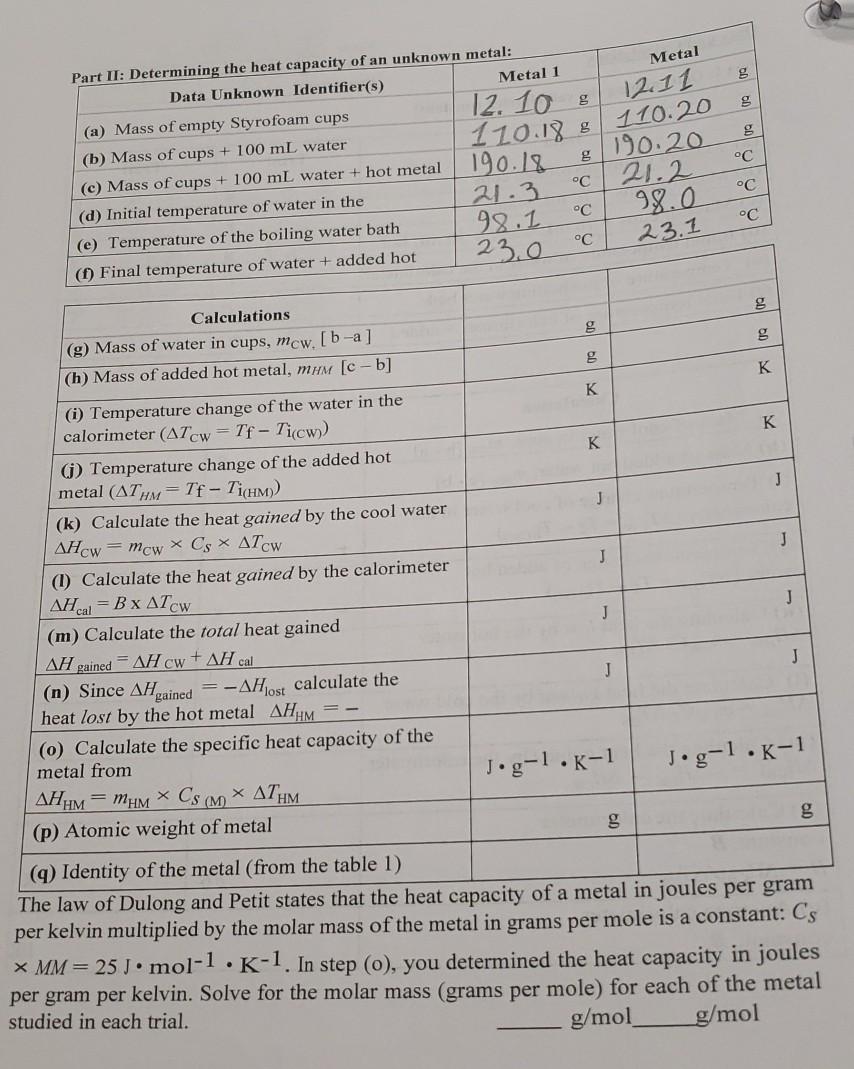
Data and Calculations Partner(s) Part 1: Determining the calorimeter constant: Trial Trial g 60 60 g g Data (a) Mass of empty Styrofoam cups (b) Mass of cups + 70 mL water (c) Mass of cups + 70 mL water + 30 mL hot (d) Initial temperature of water in the calorimeter (e) Temperature of the boiling water bath (1) Final temperature of calorimeter + added C 8.6 76.6 103.9 18.5 98.1 39.8 8.6 777 104.0 21.0 98.1 Yo.6 C C C C C Calculation 00 - g 5 09 1 K K + + - (g) Mass of cool water in cups, mcw [b-a] (h) Mass of added hot water, muw [c-b (i) Temperature change of cool water in the calorimeter (ATCW Tf - Ticw) (1) Temperature change of added hot water (AThw = Tf - Tichw)) (k) Calculate the heat lost by the hot water AHHw = mhwCs ATHW K K J - - J J + + J + AHCW (1) Calculate the heat gained by the cold water AHcw = mcwCs ATCW (m) Calculate the heat gained by the calorimeter AHcal = -AHHW (n) Calculate the calorimeter constant, B B=AHcal/ATCW (0) Average calorimeter constant, B J/K J/K J/K g Metal 12.11 110.20 g 190.20 C 98.0 23.1 Part II: Determining the heat capacity of an unknown metal: Data Unknown Identifier(s) Metal 1 (a) Mass of empty Styrofoam cups 12.10 (b) Mass of cups + 100 ml water 120.188 (c) Mass of cups + 100 mL water + hot metal 190.18 (d) Initial temperature of water in the 21.3 (e) Temperature of the boiling water bath 98.1 C (1) Final temperature of water + added hot 23.0 C c 21.2 C oc 0909 0909 K - - 1 K K K J 1 J - - J J Calculations (g) Mass of water in cups, mcw. [b-a] (h) Mass of added hot metal, mm [c-b] (i) Temperature change of the water in the calorimeter (ATcw = Tf - Ticw)) (j) Temperature change of the added hot metal (ATHM = Tf - TicHM)) (k) Calculate the heat gained by the cool water AHcw = mcw X Cs * ATCW (1) Calculate the heat gained by the calorimeter AHcal = B x ATCW (m) Calculate the total heat gained AH gained = AH CW + AH cal -AHost calculate the heat lost by the hot metal AH M = - (o) Calculate the specific heat capacity of the metal from H = x Cs ( x ATH (p) Atomic weight of metal - J J J J (n) Since AH gained Jg-1.K-1 Jg-1.K-1 g. (q) Identity of the metal (from the table 1) The law of Dulong and Petit states that the heat capacity of a metal in joules per gram per kelvin multiplied by the molar mass of the metal in grams per mole is a constant: Cs * MM = 25 J. mol-1. K-1. In step (o), you determined the heat capacity in joules per gram per kelvin. Solve for the molar mass (grams per mole) for each of the metal studied in each trial. g/mol g/mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


