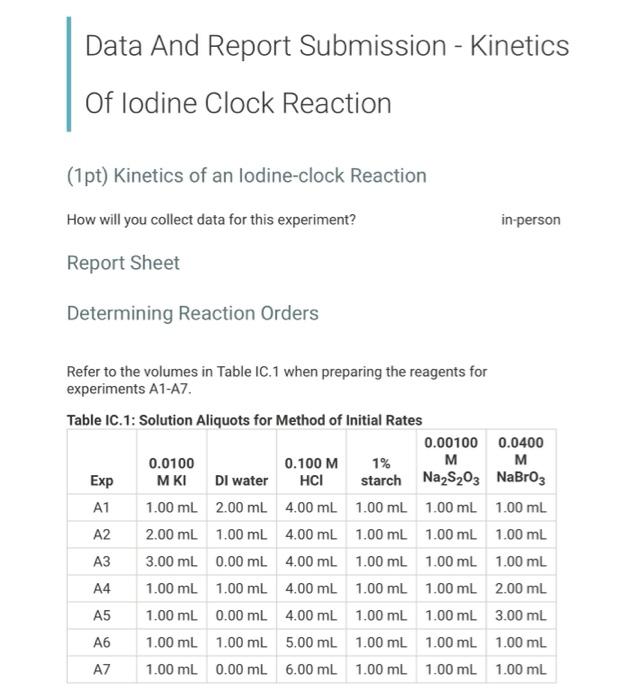
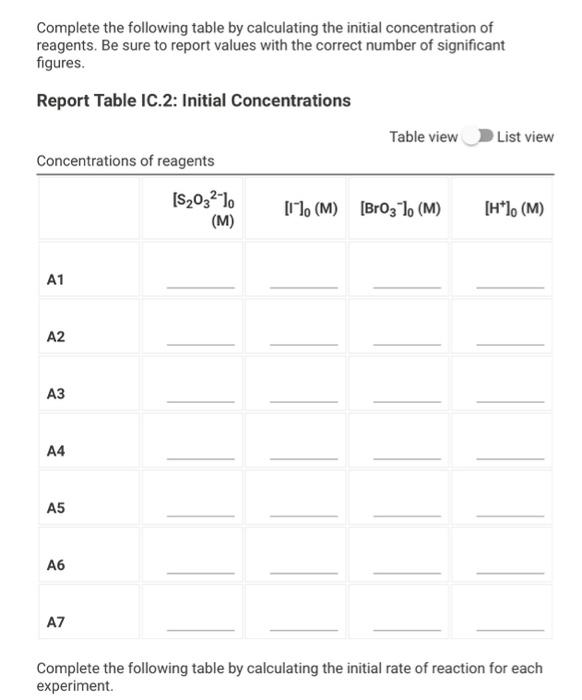
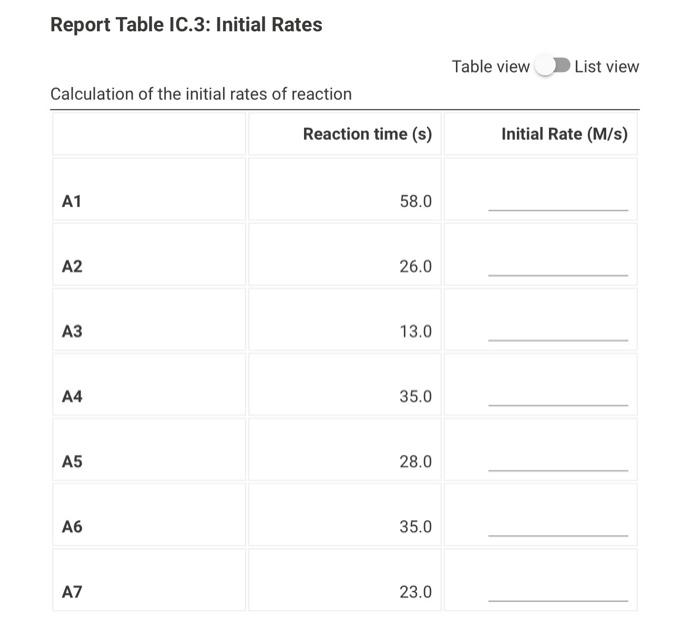
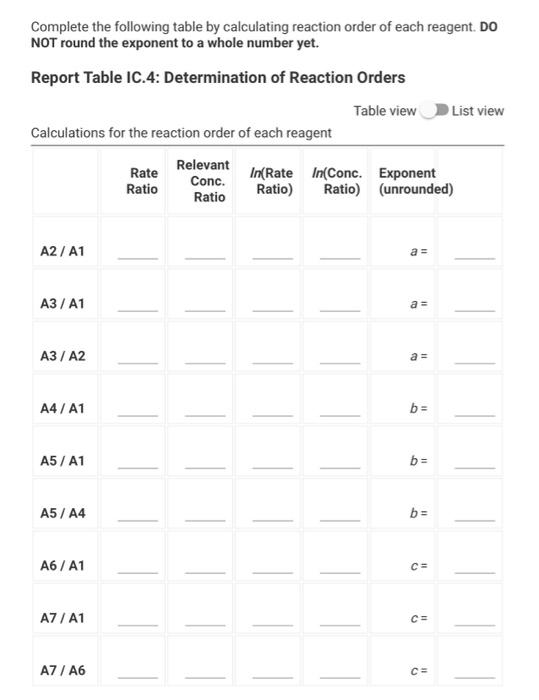
Data And Report Submission - Kinetics Of lodine Clock Reaction (1pt) Kinetics of an lodine-clock Reaction How will you collect data for this experiment? in-person Report Sheet Determining Reaction Orders Refer to the volumes in Table IC. 1 when preparing the reagents for experiments A1-A7. Table IC.1: Solution Aliquots for Method of Initial Rates 0.00100 0.0400 0.0100 0.100 M 1% M M Exp MKI Dl water HCI starch Na2S2O3 NaBroz A1 1.00 mL 2.00 mL 4.00 mL 1.00 ml 1.00 ml 1.00 mL A2 2.00 ml 1.00 mL 4.00 mL 1.00 ml 1.00 ml 1.00 mL A3 3.00 mL 0.00 mL 4.00 mL 1.00 ml 1.00 ml 1.00 mL A4 1.00 ml 1.00 mL 4.00 mL 1.00 ml 1.00 mL 2.00 mL A5 1.00 mL 0.00 mL 4.00 ml 1.00 ml 1.00 mL 3.00 mL A6 1.00 ml 1.00 mL 5.00 ml 1.00 ml 1.00 ml 1.00 mL AZ 1.00 mL 0.00 mL 6.00 mL 1.00 ml 1.00 ml 1.00 mL Temperature of reagents (C) 21.9 Note: Most stop-watches either report time in the format minutes:seconds or in the format minutes:seconds:hundredths of a second. You will be entering time in seconds. For example if a stop-watch that reported time to the second read 01:42, the time would be reported as 102 seconds. Whereas if a stop- watch that reported time to the hundreths of a second read 01:42:01, the time would be reported as 102.01 seconds. Report Table IC.1: Reaction Times Reaction times for experiments A1-A7 Reaction time (s) A1 58 A2 26 13 A4 35 A5 28 35 AZ 23 Complete the following table by calculating the initial concentration of reagents. Be sure to report values with the correct number of significant figures. Report Table IC.2: Initial Concentrations Table view List view Concentrations of reagents [S2032"). [1-10 (M) (Broz 1. (M) [H*l. (M) (M) A1 A2 A3 A4 A5 A6 A7 Complete the following table by calculating the initial rate of reaction for each experiment. Report Table IC.3: Initial Rates Table view List view Calculation of the initial rates of reaction Reaction time (s) Initial Rate (M/s) A1 58.0 A2 26.0 13.0 A4 35.0 A5 28.0 A6 35.0 A7 23.0 Complete the following table by calculating reaction order of each reagent. Do NOT round the exponent to a whole number yet. Report Table IC.4: Determination of Reaction Orders Table view List view Calculations for the reaction order of each reagent Rate Relevant Ratio Conc. In(Rate In(Conc. Exponent Ratio Ratio) Ratio) (unrounded) A2/A1 a = A3/A1 a= A3 / A2 a = A4/A1 b= = A5 / A1 bu = A5 / A4 b= 23 A6/A1 C= A7/A1 CE A7 / A6 CE Report Table IC.5: Reaction Orders Table view List view Calculations for the average and rounded exponents for each reagent Average Value of Exponent Average Value (unrounded) Rounded to an Integer a a b (6 pts) Write the rate law for the iodine-clock reaction. Normal : BIILU X 1 X TX (2pts) Give the units that the rate constant must have based on your rate law. Normal . BII U Xx X BET lli Report Table IC.6: Rate Constants at Room Temperature Table view List view Calculation of the rate constants at room temperature Rate Constant A1 A2 A4 A5 A6 AZ (1pts) Average value of rate constant: k = (4pts) Calculation Uploads