Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Data: g=9.81ms2,R=8.314103kJmol1K1 Figure Q1.1: Schematic for methane liquefaction. Methane gas is to be liquefied using the process shown in Figure Q1.1. The methane feed o
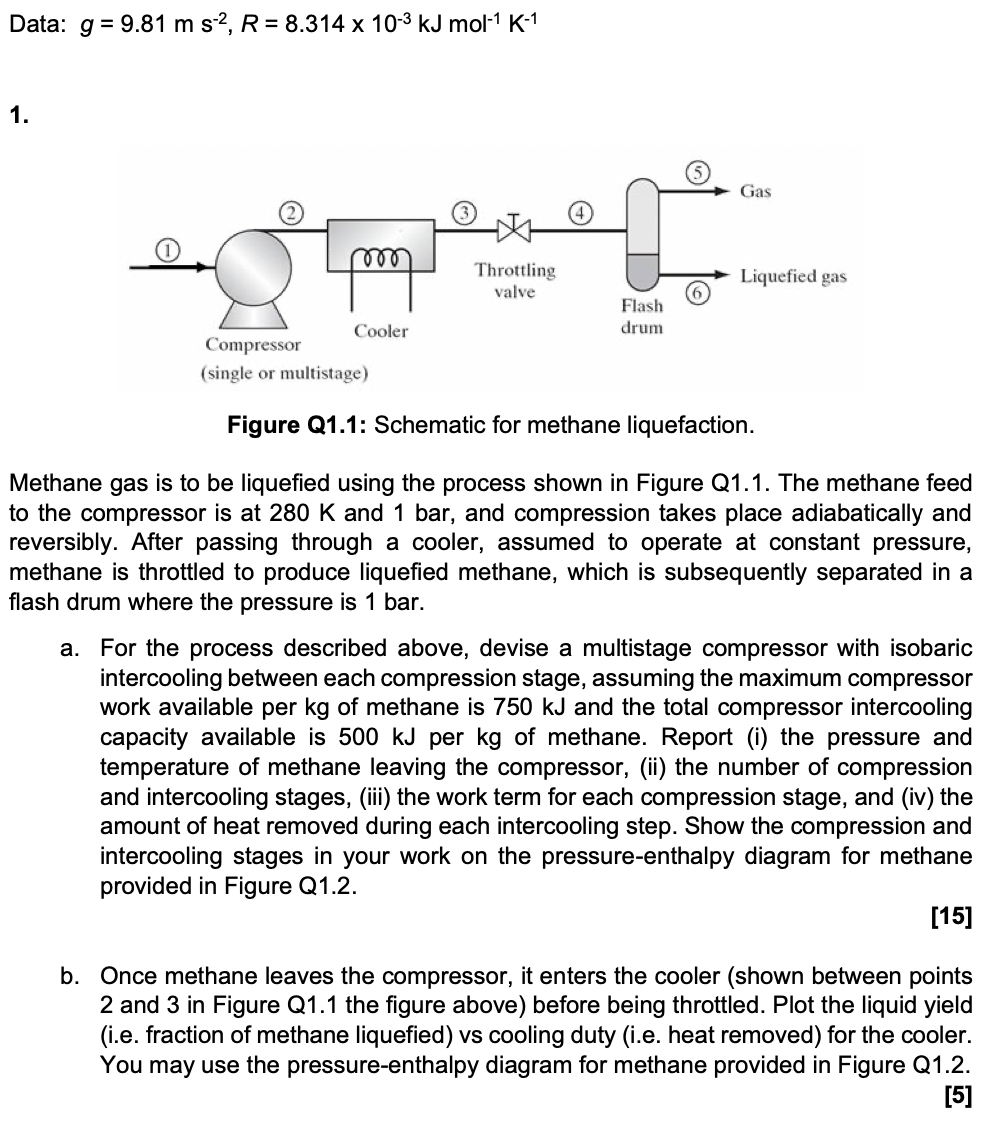
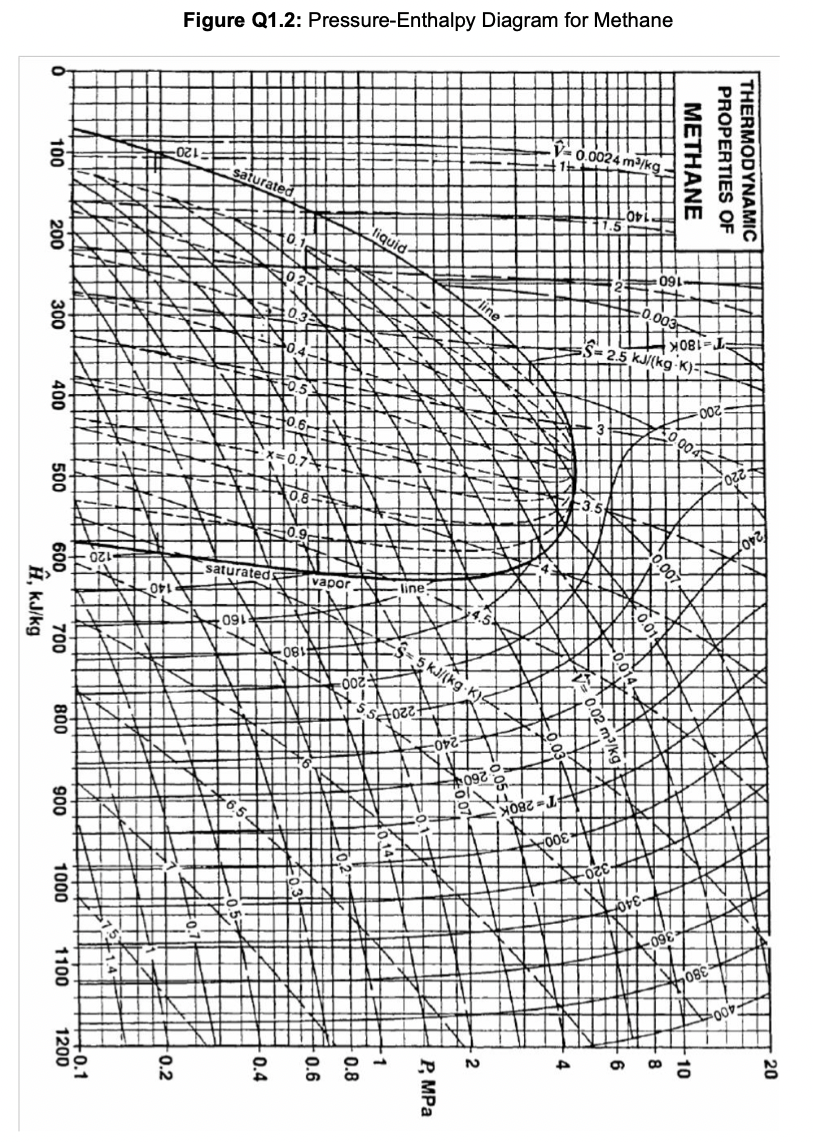
Data: g=9.81ms2,R=8.314103kJmol1K1 Figure Q1.1: Schematic for methane liquefaction. Methane gas is to be liquefied using the process shown in Figure Q1.1. The methane feed o the compressor is at 280K and 1 bar, and compression takes place adiabatically and eversibly. After passing through a cooler, assumed to operate at constant pressure, nethane is throttled to produce liquefied methane, which is subsequently separated in a lash drum where the pressure is 1 bar. a. For the process described above, devise a multistage compressor with isobaric intercooling between each compression stage, assuming the maximum compressor work available per kg of methane is 750kJ and the total compressor intercooling capacity available is 500kJ per kg of methane. Report (i) the pressure and temperature of methane leaving the compressor, (ii) the number of compression and intercooling stages, (iii) the work term for each compression stage, and (iv) the amount of heat removed during each intercooling step. Show the compression and intercooling stages in your work on the pressure-enthalpy diagram for methane provided in Figure Q1.2. [15] b. Once methane leaves the compressor, it enters the cooler (shown between points 2 and 3 in Figure Q1.1 the figure above) before being throttled. Plot the liquid yield (i.e. fraction of methane liquefied) vs cooling duty (i.e. heat removed) for the cooler. You may use the pressure-enthalpy diagram for methane provided in Figure Q1.2. [5]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


