Answered step by step
Verified Expert Solution
Question
1 Approved Answer
data Part 1. Determining the Heat Capacity of the Calorimeter Trial 1 mass of the hot water in the flask (9) 29.808 Initial temperature of
data 
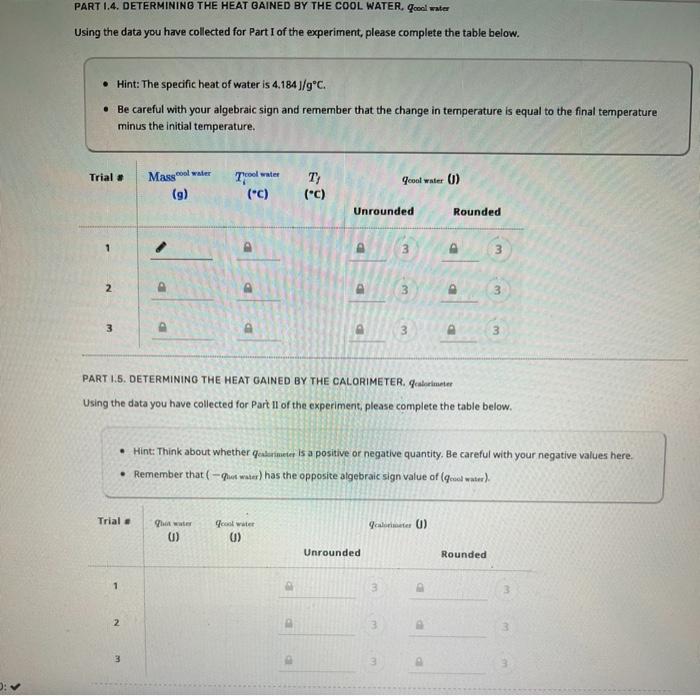
Part 1. Determining the Heat Capacity of the Calorimeter Trial 1 mass of the hot water in the flask (9) 29.808 Initial temperature of the hot water in the flask (*C) 101.7 final temperature of water in the calorimeter ("C) 45.2 mass of the cool water in the calorimeter (9) 68.700 initial temperature of the cool water in the calorimeter ("C) 23.9 Trial 2 29.178 99.9 42.9 66 300 21.7 Trial 3 29.433 100.8 43.8 67.000 22.7 PART 1.4. DETERMINING THE HEAT GAINED BY THE COOL WATER. Cool water Using the data you have collected for Part I of the experiment, please complete the table below. Hint: The specific heat of water is 4.184]/gC. Be careful with your algebraic sign and remember that the change in temperature is equal to the final temperature minus the initial temperature. Trial # Mass water 7cool water Geool water 6) (9) Ty (*C) (c) Unrounded Rounded 3 3 2 3 3 3 3 3 PART 1.5. DETERMINING THE HEAT GAINED BY THE CALORIMETER. Geslo Using the data you have collected for Part Il of the experiment, please complete the table below. Hint: Think about whether qerimeter is a positive or negative quantity. Be careful with your negative values here. Remember that-qut water) has the opposite algebraic sign value of (water) Trial. Chi wa 0) cool water 0) Scale ) Unrounded Rounded 1 3 2 3 3 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


