Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Data table 1: For each trial, the contents listed for each flask were placed into their respective flask. Each flask was then placed into a
Data table 1: For each trial, the contents listed for each flask were placed into their respective flask. Each flask was then placed into a 21.5 degrees celsius water bath, then Flask B was poured into Flask A. Data table 2 then shows the time elapsed for a change in color of the mixed solution (from clear to black). Please assist with data table and post lab questions. Thank you for your help. 



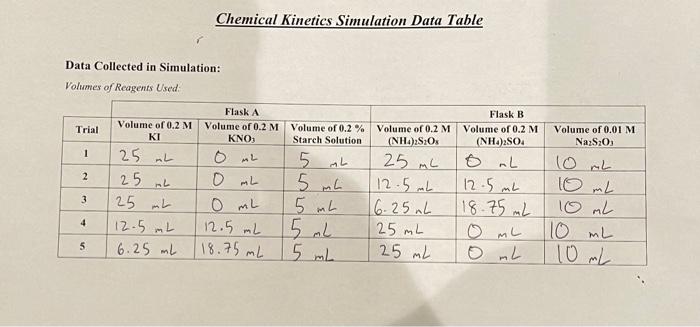
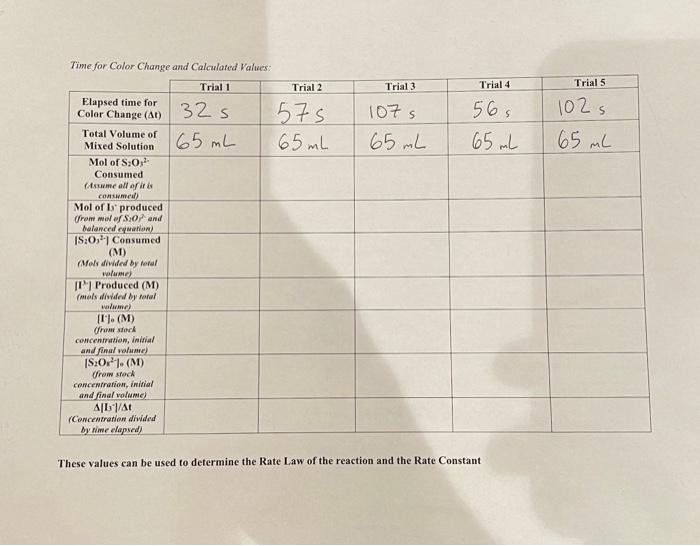

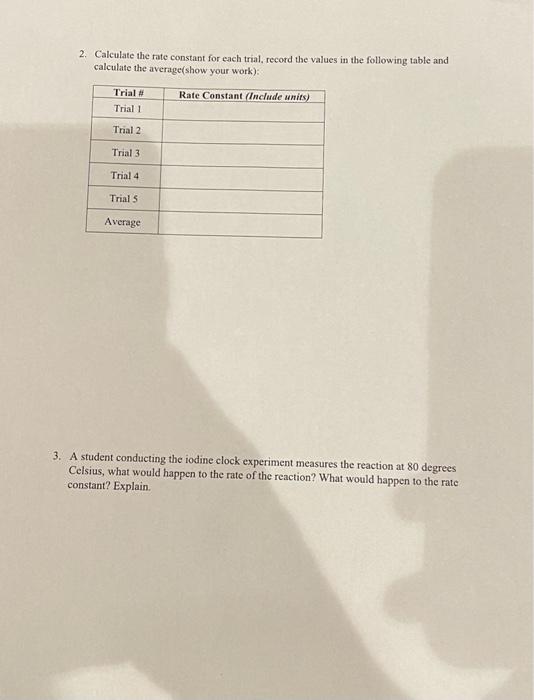
About This Lab In this lab, you will measure the rate of the reaction between iodide ion (1-) and persulfate (peroxodisulfate) ion (S,O2-). Reaction 1: 31 (aq) +S, 03 - (aq) 15 (aq) + 2 502 - (aq) To measure the rate of the reaction, you will measure the amount of I, formed in a given amount of time. Then, in reaction 2, the ls produced in reaction 1 will react with the thiosulfate ion (S,O,2-) to re-form I. Reaction 2: 15 (aq) +25,03 - (aq) -131 (aq) +S,O2 (aq) The experiment is set up so that the concentration of [S,O2-) is significantly greater than the concentration of [S,O2-). Thus, thiosulfate gets used up first. When all the thiosulfate is gone, only a small amount of persulfate has been used, so its concentration does not change appreciably during this part of the reaction. After all the thiosulfate is gone, triiodide begins to accumulate. Starch is often used in redox reactions as an indicator where triiodide is present, so the formation of triiodide is detected using starch. Triiodide forms a deep-blue complex with starch as shown in reaction 3. Reaction 3: 17 (aq) + starch(aq) [13-starch] The starch-iodide complex has a blue-black color that can be observed visually. Because the color only forms after all thiosulfate has been used up, there is a lag between the start of the experiment and color formation. Reaction 2 and reaction 3 are fast relative to reaction 1, so the time it takes for color to form can be used as a clock to time reaction 1. The amount of triiodide formed at the time the color change occurs can be determined from the balanced chemical equation for reaction 2. For two moles of thiosulfate reacted, one mole of triiodide is formed. Thus, the molar concentration of 1, formed and the rate of reaction (A[13]/At) can be calculated. The value of p can be determined by plotting log(rate) versus log[(-) at constant [S2002-), and a plot of log(rate) versus log[S,0,2-) at constant [-] can be used to determine q. The values for p and q are used in the rate lequation to determine the rate constant for the rate law. rate = k[I-I'S,%- Ionic strength is a measure of the concentration of ions in solution. For ionic reactions, variations in ionic strength can significantly affect the rate of reaction. In this experiment, the rate of an ionic reaction is measured at different concentrations of each reactant. To maintain a constant ionic strength, inert solutions containing the same counter ion will be added a neador Cor Tonic reaction is measured at different concentrations of each reactant. To maintain a constant ionic strength, inert solutions containing the same counter ion will be added as needed. For example, KNO, will be added to compensate for differences in the concentration of Kl present. Each compound dissociates in solution as shown. KI K+ + I KNO, K+ + NOZ Both reactions yield one positively charged ion (K*) and one negatively charged ion (l- or NO3-). Thus, if the total concentration of Kl and KNO, present in the reaction mixture is held constant, each reaction mixture will have the same ionic strength. Likewise, (NH4)2SO, will be added to compensate for differences in the concentration of (NH., S,O, present. Each compound dissociates in solution as shown. - (NH4)2S20g 2 NH3 + 8,0 (NH4)2SO4 2 NHI + SO2- Both reactions yield two positively charged ions (2NH4+) and a single ion with a charge of minus two (S,0,2- or SO 2-). Again, ionic strength can be held constant by adding the same total concentration of each compound to each reaction mixture. Finally, you will determine the rate of the reaction below. Finally, you will determine the rate of the reaction below. rate = k[1![S,03-1 where p, the order of the reaction with respect to [1], will be determined by measuring the rate at different [1] and constant (S,0,2-); q, the order of the reaction with respect to [S,0,2-1, will be determined by measuring the rate at different [S, 0,2-) and constant (l). Once you have determied the values of p and q, you will solve the rate equation to determine the value of k. Chemical Kinetics Simulation Data Table Volume of 0.2% Starch Solution Volume of 0.01 M NazS20 Data Collected in Simulation: Volumes of Reagents Used Flask A Volume of 0.2 M Volume of 0.2 M Trial KNO, 1 AL 2 25 mL 3 25 0 mL 12.5 mL 12.5 mL 5 6.25 ml 18.75 mL 25 mL 5 25 mL Flask B Volume of 0.2 M Volume of 0.2 M (NH4)2S:Os (NH4)2SO4 0 mL 12.5 mL 12.5 mL 6.25nL 18.75 mL 25 mL ML 25 ml 0 mL 5 mL 5 L 15 mL 5 mL 10 mL 10 mL 10 mL 10 mL 10mL 4 4 Time for Color Change and Calculated Values: Trial 1 Trial 2 Trial 3 Trial 4 Trials 32 s 575 65 mL 107s 65 mL 56s 65 mL 102 s 65 mL 165 mL Elapsed time for Color Change (At) Total Volume of Mixed Solution Mol of S:0,- Consumed (Aswame all of its Consumeal) Mol of ls produced from malef S:0 and Malanced cywallow) 19:0,"Consumed (M) (Mols diwided by total wolume) 1 Produced (M) (mals diwded by total valamel [I"). (M) from stock concentration, initial and final wame) IS:0). (M) from stock concentration, initial and final volume) A1/A (Concentration divided by time elapsed) These values can be used to determine the Rate Law of the reaction and the Rate Constant Calculations and Post Lab Questions: 1. Determine the Rate Law for the reaction using the initial rate and concentrations determined in the table above (Show your work): FOR FOR 1 Reaction trial 2. Calculate the rate constant for each trial, record the values in the following table and calculate the average(show your work) Trial # Trial 1 Rate Constant (include units) Trial 2 Trial 3 Trial 4 Trials Average 3. A student conducting the iodine clock experiment measures the reaction at 80 degrees Celsius, what would happen to the rate of the reaction? What would happen to the rate constant? Explain. About This Lab In this lab, you will measure the rate of the reaction between iodide ion (1-) and persulfate (peroxodisulfate) ion (S,O2-). Reaction 1: 31 (aq) +S, 03 - (aq) 15 (aq) + 2 502 - (aq) To measure the rate of the reaction, you will measure the amount of I, formed in a given amount of time. Then, in reaction 2, the ls produced in reaction 1 will react with the thiosulfate ion (S,O,2-) to re-form I. Reaction 2: 15 (aq) +25,03 - (aq) -131 (aq) +S,O2 (aq) The experiment is set up so that the concentration of [S,O2-) is significantly greater than the concentration of [S,O2-). Thus, thiosulfate gets used up first. When all the thiosulfate is gone, only a small amount of persulfate has been used, so its concentration does not change appreciably during this part of the reaction. After all the thiosulfate is gone, triiodide begins to accumulate. Starch is often used in redox reactions as an indicator where triiodide is present, so the formation of triiodide is detected using starch. Triiodide forms a deep-blue complex with starch as shown in reaction 3. Reaction 3: 17 (aq) + starch(aq) [13-starch] The starch-iodide complex has a blue-black color that can be observed visually. Because the color only forms after all thiosulfate has been used up, there is a lag between the start of the experiment and color formation. Reaction 2 and reaction 3 are fast relative to reaction 1, so the time it takes for color to form can be used as a clock to time reaction 1. The amount of triiodide formed at the time the color change occurs can be determined from the balanced chemical equation for reaction 2. For two moles of thiosulfate reacted, one mole of triiodide is formed. Thus, the molar concentration of 1, formed and the rate of reaction (A[13]/At) can be calculated. The value of p can be determined by plotting log(rate) versus log[(-) at constant [S2002-), and a plot of log(rate) versus log[S,0,2-) at constant [-] can be used to determine q. The values for p and q are used in the rate lequation to determine the rate constant for the rate law. rate = k[I-I'S,%- Ionic strength is a measure of the concentration of ions in solution. For ionic reactions, variations in ionic strength can significantly affect the rate of reaction. In this experiment, the rate of an ionic reaction is measured at different concentrations of each reactant. To maintain a constant ionic strength, inert solutions containing the same counter ion will be added a neador Cor Tonic reaction is measured at different concentrations of each reactant. To maintain a constant ionic strength, inert solutions containing the same counter ion will be added as needed. For example, KNO, will be added to compensate for differences in the concentration of Kl present. Each compound dissociates in solution as shown. KI K+ + I KNO, K+ + NOZ Both reactions yield one positively charged ion (K*) and one negatively charged ion (l- or NO3-). Thus, if the total concentration of Kl and KNO, present in the reaction mixture is held constant, each reaction mixture will have the same ionic strength. Likewise, (NH4)2SO, will be added to compensate for differences in the concentration of (NH., S,O, present. Each compound dissociates in solution as shown. - (NH4)2S20g 2 NH3 + 8,0 (NH4)2SO4 2 NHI + SO2- Both reactions yield two positively charged ions (2NH4+) and a single ion with a charge of minus two (S,0,2- or SO 2-). Again, ionic strength can be held constant by adding the same total concentration of each compound to each reaction mixture. Finally, you will determine the rate of the reaction below. Finally, you will determine the rate of the reaction below. rate = k[1![S,03-1 where p, the order of the reaction with respect to [1], will be determined by measuring the rate at different [1] and constant (S,0,2-); q, the order of the reaction with respect to [S,0,2-1, will be determined by measuring the rate at different [S, 0,2-) and constant (l). Once you have determied the values of p and q, you will solve the rate equation to determine the value of k. Chemical Kinetics Simulation Data Table Volume of 0.2% Starch Solution Volume of 0.01 M NazS20 Data Collected in Simulation: Volumes of Reagents Used Flask A Volume of 0.2 M Volume of 0.2 M Trial KNO, 1 AL 2 25 mL 3 25 0 mL 12.5 mL 12.5 mL 5 6.25 ml 18.75 mL 25 mL 5 25 mL Flask B Volume of 0.2 M Volume of 0.2 M (NH4)2S:Os (NH4)2SO4 0 mL 12.5 mL 12.5 mL 6.25nL 18.75 mL 25 mL ML 25 ml 0 mL 5 mL 5 L 15 mL 5 mL 10 mL 10 mL 10 mL 10 mL 10mL 4 4 Time for Color Change and Calculated Values: Trial 1 Trial 2 Trial 3 Trial 4 Trials 32 s 575 65 mL 107s 65 mL 56s 65 mL 102 s 65 mL 165 mL Elapsed time for Color Change (At) Total Volume of Mixed Solution Mol of S:0,- Consumed (Aswame all of its Consumeal) Mol of ls produced from malef S:0 and Malanced cywallow) 19:0,"Consumed (M) (Mols diwided by total wolume) 1 Produced (M) (mals diwded by total valamel [I"). (M) from stock concentration, initial and final wame) IS:0). (M) from stock concentration, initial and final volume) A1/A (Concentration divided by time elapsed) These values can be used to determine the Rate Law of the reaction and the Rate Constant Calculations and Post Lab Questions: 1. Determine the Rate Law for the reaction using the initial rate and concentrations determined in the table above (Show your work): FOR FOR 1 Reaction trial 2. Calculate the rate constant for each trial, record the values in the following table and calculate the average(show your work) Trial # Trial 1 Rate Constant (include units) Trial 2 Trial 3 Trial 4 Trials Average 3. A student conducting the iodine clock experiment measures the reaction at 80 degrees Celsius, what would happen to the rate of the reaction? What would happen to the rate constant? Explain 







Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


