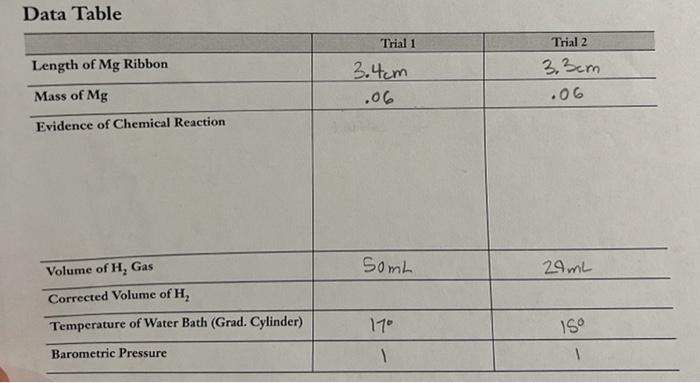
Data Table \begin{tabular}{l|c|c} \hline Length of Mg Ribbon & Trial 1 & Trial 2 \\ \hline Mass of Mg & 3.4cm & 3.3cm \\ \hline Evidence of Chemical Reaction & .06 & .06 \\ \hline & & \\ \hline Volume of H2 Gas & & \\ \hline Corrected Volume of H2 & 50mL & 24mL \\ \hline Temperature of Water Bath (Grad. Cylinder) & 11 & 150 \\ \hline Barometric Pressure & 1 & 1 \\ \hline \end{tabular} 2. Use Table 1 in the Background section to find the vapor pressure of water at the temperature of the water bath in this experiment. Calculate the partial pressure of hydrogen gas produced in Trials 1 and 2. 3. Use the combined gas law to convert the measured volume of hydrogen to the "ideal" volume the hydrogen gas would occupy at STP for Trials 1 and 2. Hint: Remember the units. 4. Divide the volume of hydrogen gas at STP by the theoretical number of moles of hydrogen to calculate the molar volume of hydrogen for Trials 1 and 2 . 5. What is the average value of the molar volume of hydrogen2 Look up the literature value of the molar volume of a gas in your textbook and calculate the percent error in your experimental determination of the molar volume of hydrogen. Percenterror=Literaturevalue|ExperimentalvalueLiteraturevalueI100% 6. One mole of hydrogen gas has a mass of 2,02g. Use your value of the molar volume of hydrogen to calculate the mass of one liter of hydrogen gas at STP. This is the density of hydrogen in g/L. How does this experimental value of the density compare with the literature value? (Consult a chemistry handbook for the density of hydrogen.) 7. In setting up this experiment, a student noticed that a bubble of air leaked into the graduated cylinder when it was inverted in the water bath. What effect would this have on the measured volume of hydrogen gas? Would the calculated molar volume of hydrogen be too high or too low as a result of this error? Explain. 8. A student noticed that the magnesium ribbon appeared to be oxidized-the metal surface was black and dull rather than silver and shiny. What effect would this error have on the measured volume of hydrogen gas? Would the calculated molar volume of hydrogen be too high or too low as a result of this error? Explain