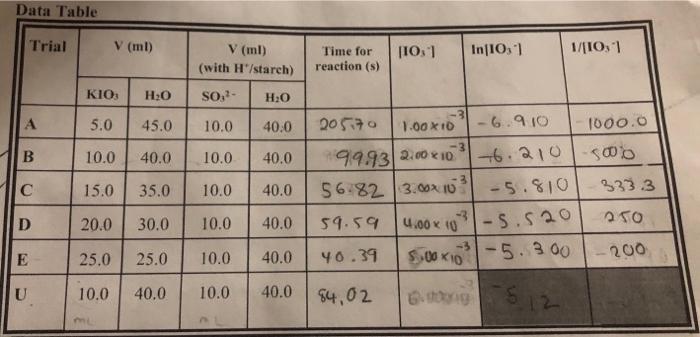
Data Table Trial V (ml) In 10,1 V (ml) (with H/starch) 110,1 1/10,1 Time for reaction (s) KIO H2O SO, H:0 A 5.0 45.0 10.0 40.0 3 B 10.0 40.0 10.0 40.0 15.0 35.0 10.0 40.0 D 20.0 30.0 10.0 40.0 205170 1.00 x 10 -6.910 1000.0 99.932.00 210 6.210 5000 56.82 3.00x103 -5.810 3333 59.59 -5.520 40.39 5.00x1031-5.300 - 5.300 - 200 84,02 B0512 4.00 10 E 25.0 25.0 10.0 40.0 U 10.0 40.0 10.0 40.0 1) Use Excel to prepare the three plots: "quantity" vs. time, and circle the correct order reaction. Ensure that graphs are labeled properly and that you indicate the correlation factor for linearity on each graph. Zero-order reaction equation and R value from the plot: First-order reaction equation and R value from the plot: Second-order reaction equation and value from the plot: CHEM 1877 Lab Manual 2) (a) Write down a general Rate Law and Integrated Rate Law (b) Determine the rate constant (c) Determine the half-life for this reaction it [A] +0.025 M. 3) Based on the linear plot of the correct order in part (1) (a) Determine the y value at the time of unknown solution observed from the plot (b) Determine the concentration of the original unknown using the y value: Data Table Trial V (ml) In 10,1 V (ml) (with H/starch) 110,1 1/10,1 Time for reaction (s) KIO H2O SO, H:0 A 5.0 45.0 10.0 40.0 3 B 10.0 40.0 10.0 40.0 15.0 35.0 10.0 40.0 D 20.0 30.0 10.0 40.0 205170 1.00 x 10 -6.910 1000.0 99.932.00 210 6.210 5000 56.82 3.00x103 -5.810 3333 59.59 -5.520 40.39 5.00x1031-5.300 - 5.300 - 200 84,02 B0512 4.00 10 E 25.0 25.0 10.0 40.0 U 10.0 40.0 10.0 40.0 1) Use Excel to prepare the three plots: "quantity" vs. time, and circle the correct order reaction. Ensure that graphs are labeled properly and that you indicate the correlation factor for linearity on each graph. Zero-order reaction equation and R value from the plot: First-order reaction equation and R value from the plot: Second-order reaction equation and value from the plot: CHEM 1877 Lab Manual 2) (a) Write down a general Rate Law and Integrated Rate Law (b) Determine the rate constant (c) Determine the half-life for this reaction it [A] +0.025 M. 3) Based on the linear plot of the correct order in part (1) (a) Determine the y value at the time of unknown solution observed from the plot (b) Determine the concentration of the original unknown using the y value