Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of
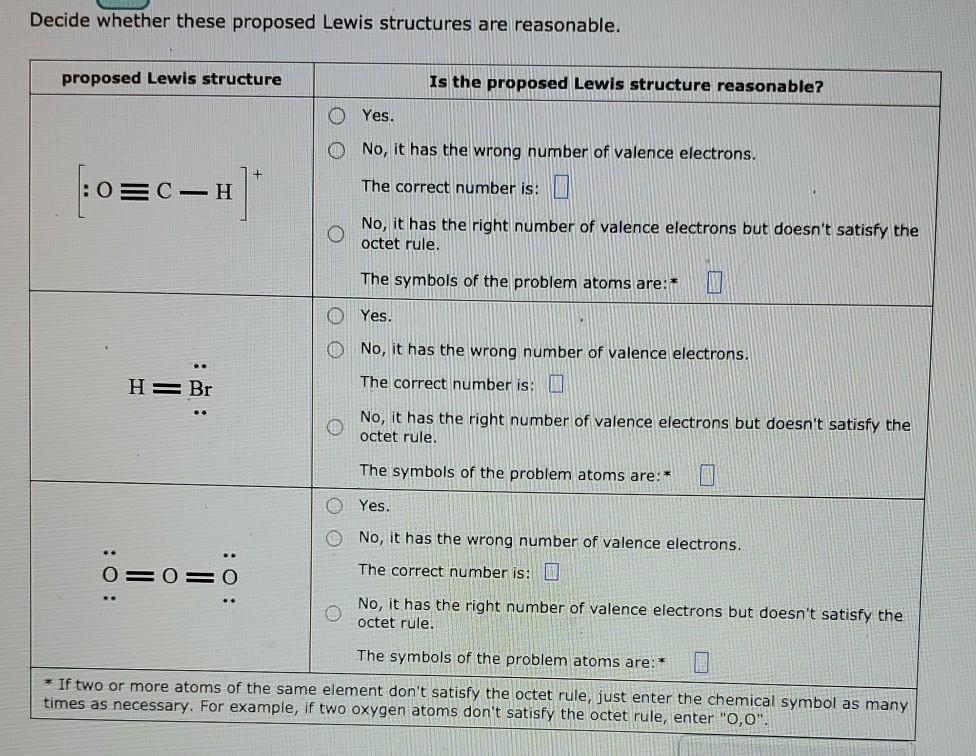
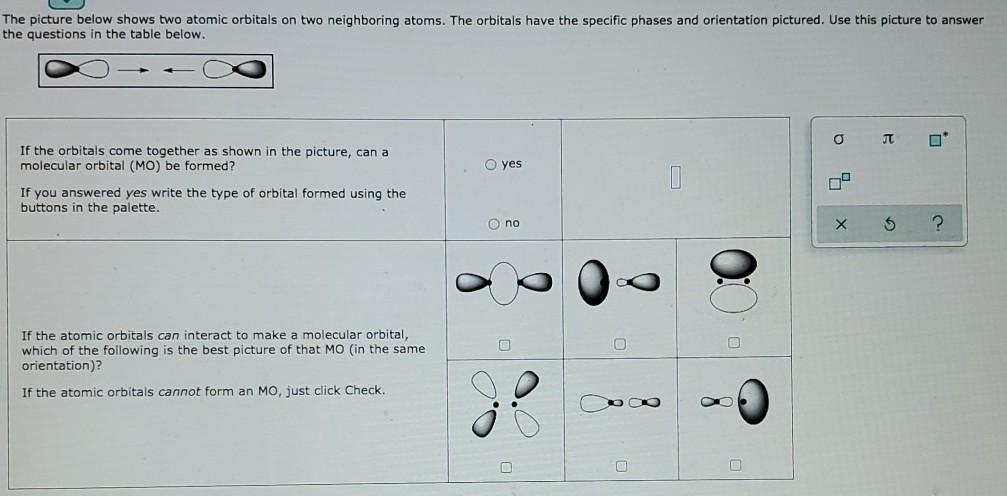
Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. : 03C-H The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* 0 Yes. O No, it has the wrong number of valence electrons. The correct number is: H= Br No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. 0 0 No, it has the wrong number of valence electrons. The correct number is: D O=O=O No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: * * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". The picture below shows two atomic orbitals on two neighboring atoms. The orbitals have the specific phases and orientation pictured. Use this picture to answer the questions in the table below. o O yes If the orbitals come together as shown in the picture, can a molecular orbital (MO) be formed? If you answered yes write the type of orbital formed using the buttons in the palette. no $ ? c o If the atomic orbitals can interact to make a molecular orbital, which of the following is the best picture of that MO (in the same orientation)? If the atomic orbitals cannot form an MO, just click Check
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


