Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Dehydrogenation of ethylbenzene to styrene is normally accomplished in a fixed-bed reactor. A catalyst is packed in tubes to form the fixed bed. Steam
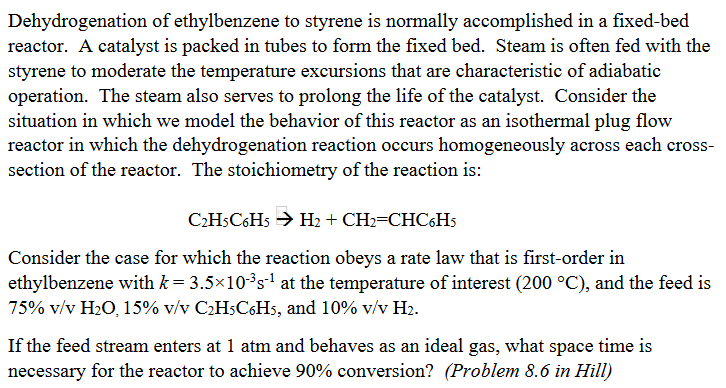
Dehydrogenation of ethylbenzene to styrene is normally accomplished in a fixed-bed reactor. A catalyst is packed in tubes to form the fixed bed. Steam is often fed with the styrene to moderate the temperature excursions that are characteristic of adiabatic operation. The steam also serves to prolong the life of the catalyst. Consider the situation in which we model the behavior of this reactor as an isothermal plug flow reactor in which the dehydrogenation reaction occurs homogeneously across each cross- section of the reactor. The stoichiometry of the reaction is: C2H5C6H5 H2 + CH2=CHC6H5 Consider the case for which the reaction obeys a rate law that is first-order in ethylbenzene with k= 3.510-s at the temperature of interest (200 C), and the feed is 75% v/v HO, 15% v/v CH5C6H5, and 10% v/v H. If the feed stream enters at 1 atm and behaves as an ideal gas, what space time is necessary for the reactor to achieve 90% conversion? (Problem 8.6 in Hill)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


