Question
M. dos Santos Afonso and H. J. Schumacher pInt. J. Chem. Kinet. 16, 103-115 (1984)] reported that the rate law for the gas-phase reaction
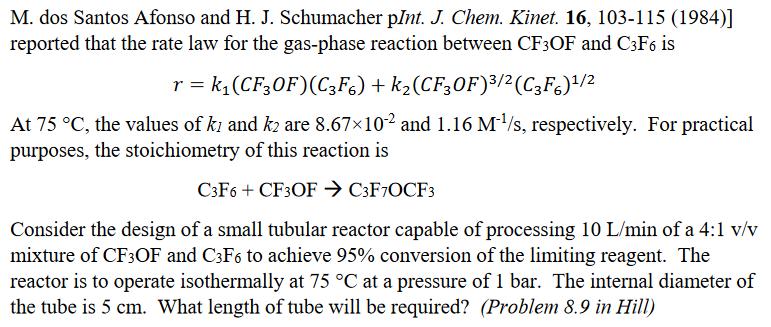
M. dos Santos Afonso and H. J. Schumacher pInt. J. Chem. Kinet. 16, 103-115 (1984)] reported that the rate law for the gas-phase reaction between CF3OF and C3F6 is r = k,(CF30F)(C3F6) + k2(CF30F)/2(C3F6)/2 At 75 C, the values of ki and k2 are 8.67x102 and 1.16 M/s, respectively. For practical purposes, the stoichiometry of this reaction is C3F6 + CF3OF > C3F7OCF3 Consider the design of a small tubular reactor capable of processing 10 L/min of a 4:1 v/v mixture of CF3OF and C3F6 to achieve 95% conversion of the limiting reagent. The reactor is to operate isothermally at 75 C at a pressure of 1 bar. The internal diameter of the tube is 5 cm. What length of tube will be required? (Problem 8.9 in Hill)
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Volume of the entire r...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



