Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Under appropriate conditions, the gas-phase partial oxidation of acetaldehyde to peracetic acid is autocatalytic. The reaction stoichiometry is CH3CHO + O2 CH3CO3H and the
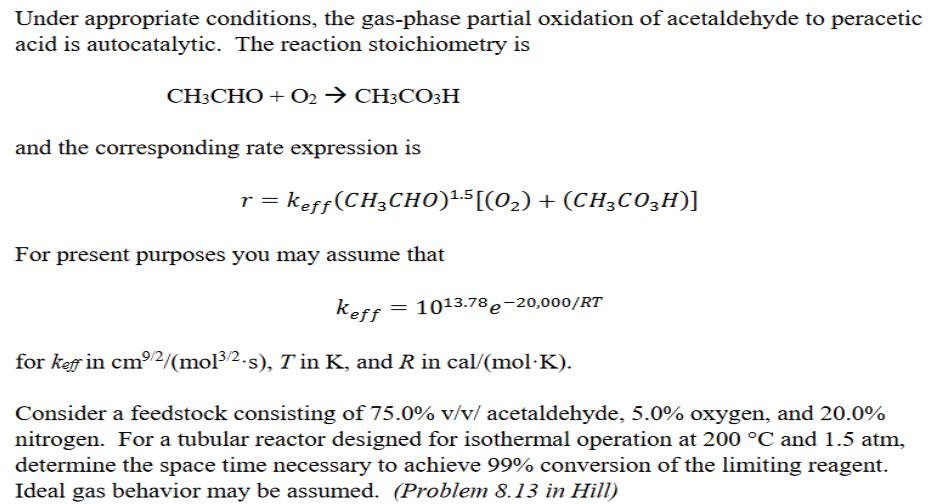

Under appropriate conditions, the gas-phase partial oxidation of acetaldehyde to peracetic acid is autocatalytic. The reaction stoichiometry is CH3CHO + O2 CH3CO3H and the corresponding rate expression is r = keff(CH3CH0)LS[(0,) + (CH3CO3H)] For present purposes you may assume that keff 1013.78 e-20,000/RT for keff in cm2/(mol3/2.s), T in K, and R in cal/(molK). Consider a feedstock consisting of 75.0% v/v/ acetaldehyde, 5.0% oxygen, and 20.0% nitrogen. For a tubular reactor designed for isothermal operation at 200 C and 1.5 atm, determine the space time necessary to achieve 99% conversion of the limiting reagent. Ideal gas behavior may be assumed. (Problem 8.13 in Hill) . The elementary, irreversible gas-phase reaction below is carried out in a packed bed reactor (PBR) containing 50 kg of catalyst. The reactor consists of 10 tubes each with an inside diameter of 2.0 inches. Pure A enters the reactor at a volumetric flow rate of 25L/s, a pressure of 10atm, and a temperature of 450K. Assuming the reactor is isothermal, determine FA as a function of W and plot FA and fa vs. W. A B+C The molecular weight of species A is 72 kg/kmol The viscosity of the gas mixture can be assumed constant at 210-5 Pa-s k(450K) = 0.133 L/(kg-cat-s) and Ea = 31.4 kJ/mol Catalyst properties: Spherical pellets: 4 mm diameter Void fraction: 0.4 Pellet density: 2,000 kg/m A. Plot fa, and P/Po vs. W (kg). B. What is FA (kmol/hr) for 50 kg of catalyst? C. You desire 60% conversion. To what temperature (in K) should you preheat the feed? Be careful not to change the molar flow rate of the feed when you change the temperature of the preheater. D. What is the length of the tubes (inches) in the reactor?
Step by Step Solution
★★★★★
3.50 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


