Answered step by step
Verified Expert Solution
Question
1 Approved Answer
deriving deriving this CSTR Derivation without integrationCSTR solve 11-3 step by step in an external sheet 2. CSTR with heal exchanger, UA (TaT), and large
deriving 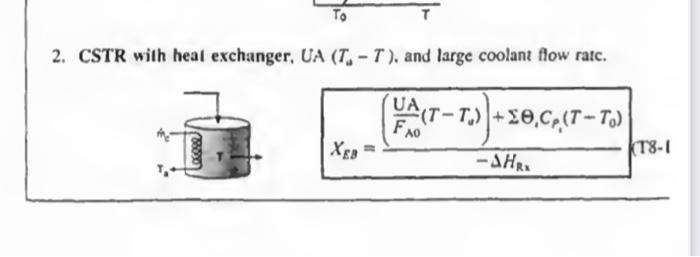
deriving this CSTR 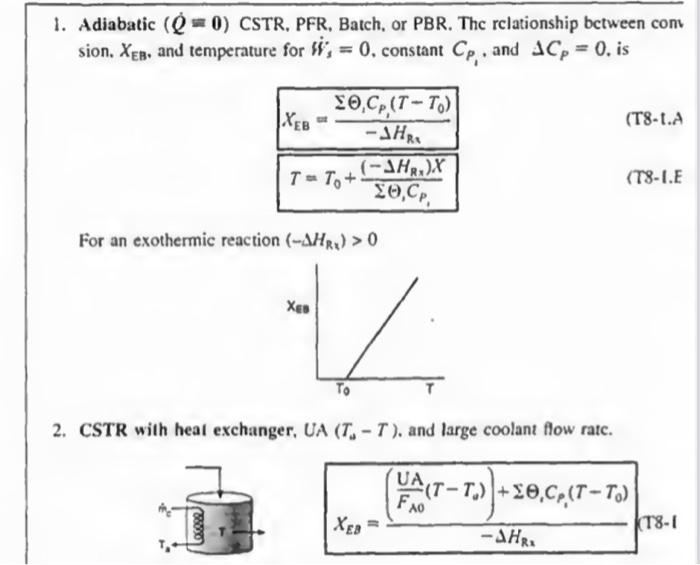
Derivation without integrationCSTR 
2. CSTR with heal exchanger, UA (TaT), and large coolant flow ratc. XEB=HRx(FA0UA(TTa))+1CP1(TT0)(T81 1. Adiabatic ( Q=0) CSTR, PFR, Batch, or PBR. The rclationship between conv sion. XEB, and temperature for Hs=0, constant CP1, and CP=0, is XEB=HRS1CP1(TT0)T=T0+CP1(HRx)X For an exothermic reaction (HRx)>0 2. CSTR with heal exchanger, UA (TaT), and large coolant flow ratc. XEB=HRI(FA0UA(TT0))+1CP1(TT0) dWdT=FA0(()iCp+CM)paUa(TsT)riHRn(T) 3B. PFR in terms of converslon 3C. PBR in terms of molar flow rates dWdT=FsCrssCg(TsT)+riHns(T) 3D. PFR in terms of molar flow rates \[ \frac{d T}{d V}=\frac{\left.U_{a}\left(T_{2}-T ight)+r_{1} ight\lrcorner H_{\mathrm{z}_{1}}(T)}{\searrow F_{1} C_{r_{3}}} \] 4. Batch dtdT=N1CP1(r1V1)(Hn1)UA(TT2) 5. For Semibatch or unsteady CSTR dtdT=i=1nNiCPiQWii=1nFi0(CP1(TTi0)+1HR1(T)](rV)) 6. For multiple reactlons in a PFR dVdT=F1Cr1Ua(T1T)+r1,HR2 tsistA lial maite of Fuatipulating the tiletar balance Set. min 824 to 8.4 For an exteermic maction (Mim4}>0 Suc. 42 The Enerer thance 477 3. P7 WYPEs with heat rurhander. 3. Fint in luras af cumatilin (Ty-1.1.) 11. PFIl in ierme of qubersion TW-LE 3C. Pat in Acrine of mular fere ratrs 3D. PFR le terms of malur the rakis (T-1G) 4. Bateh ITt. HH3 1. For bemihatch or uncirade ChTIR Example 11-3 Adiabatic Liquid-Phase Isomerization of Normal Butane Normal butane, C4H10, is to be isomerized to isobutane in a plug-flow reactor. Isobutane is a valuable product that is used in the manufacture of gasoline additives. For example, isobutane can be further reacted to form iso-octane. The 2014 selling price of n-butane was $1.5/gal, while the trading price of isobutane was $1.75/gal This elementary reversible reaction is to be carried out adiabatically in the liquid phase under high pressure using essentially trace amounts of a liquid catalyst that gives a specific reaction rate of 31.1h1 at 360K. The feed enters at 330K. (a) Calculate the PFR volume necessary to process 100,000gal/ day (163kmol/h) at 70% conversion of a mixture 90mol%n-butane and 10mol%-pentane, which is considered an inert. (b) Plot and analyze X,XeT, and rA down the length of the reactor. (c) Calculate the CSTR volume for 40% conversion solve 11-3 step by step in an external sheet 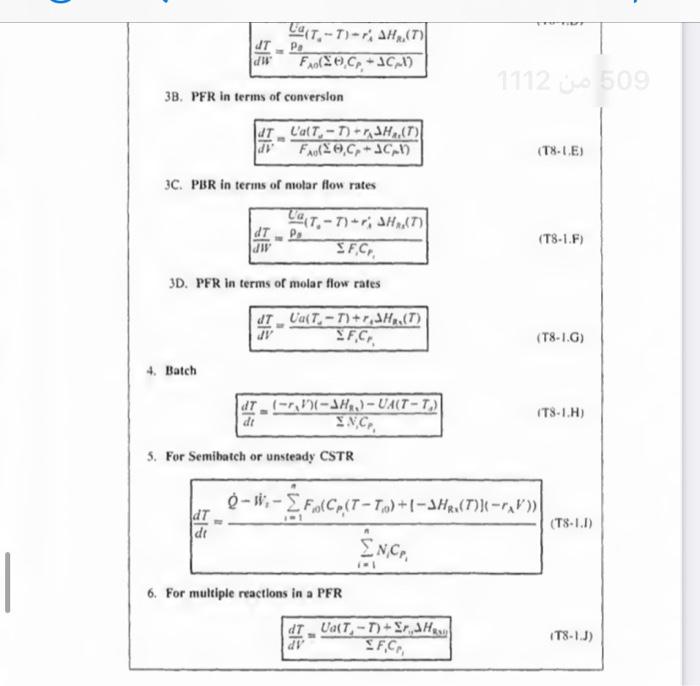




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


