Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Describe the autoacceleration and autodeceleration processes in radical polymerizations and how they are affected by crosslinking, the presence / amount of a solvent and temperature.
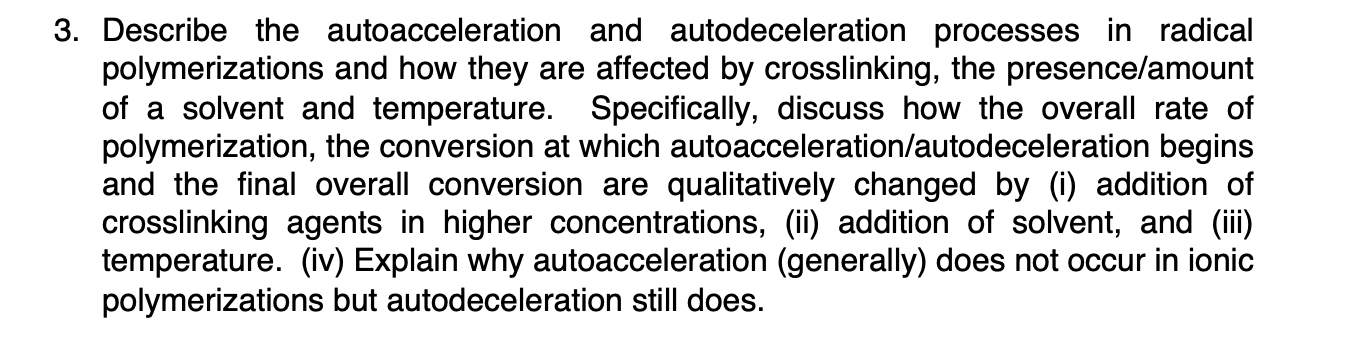
Describe the autoacceleration and autodeceleration processes in radical
polymerizations and how they are affected by crosslinking, the presenceamount
of a solvent and temperature. Specifically, discuss how the overall rate of
polymerization, the conversion at which autoaccelerationautodeceleration begins
and the final overall conversion are qualitatively changed by i addition of
crosslinking agents in higher concentrations, ii addition of solvent, and iii
temperature. iv Explain why autoacceleration generally does not occur in ionic
polymerizations but autodeceleration still does.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


