Answered step by step
Verified Expert Solution
Question
1 Approved Answer
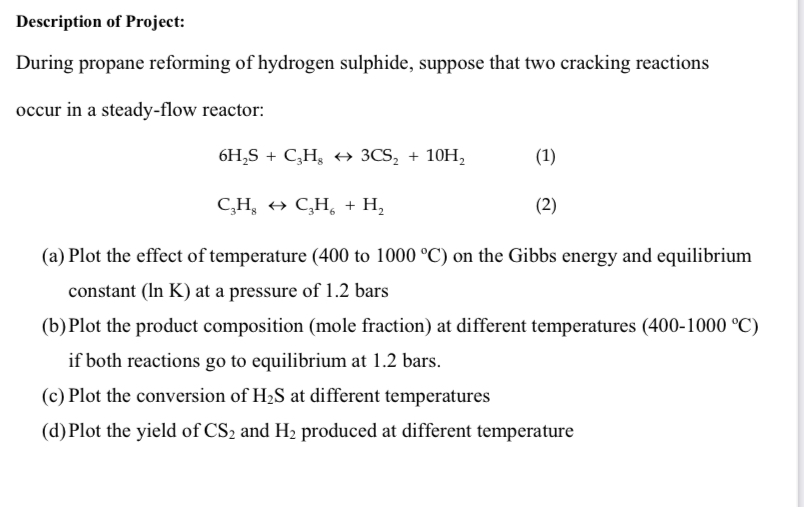
Description of Project: During propane reforming of hydrogen sulphide, suppose that two cracking reactions occur in a steady - flow reactor: 6 H 2 S
Description of Project:
During propane reforming of hydrogen sulphide, suppose that two cracking reactions
occur in a steadyflow reactor:
harr
a Plot the effect of temperature to on the Gibbs energy and equilibrium
constant at a pressure of bars
b Plot the product composition mole fraction at different temperatures
if both reactions go to equilibrium at bars.
c Plot the conversion of at different temperatures
d Plot the yield of and produced at different temperature
Solve by steps using numbers and final answer as number
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


