You will take on the role of a project manager assigned to guide a product to market effectively. Your output is a complete project plan
You will take on the role of a project manager assigned to guide a product to market effectively. Your output is a complete project plan that includes, but is not limited to, a WBS, network diagrams, Gantt Chart, and a resource loading graph. Your plan will also have a comparative analysis, a risk analysis, the project’s goals and objectives, what is in and out of scope, stakeholder analysis, and project costs and duration.
Please I need help with WBS, network diagrams, Gantt Chart, and a resource loading graph also comparative analysis, a risk analysis, the project’s goals and objectives, what is in and out of scope, stakeholder analysis, and project costs and duration. Also help me with the Excel sheet values.
TRIAD Pharmaceuticals Company has done sufficient new product development at the
research and development level to estimate a high likelihood of technical success for a product
of assured commercial success: A long-term antiseptic. Executive management has instructed
the Triad Pharmaceuticals Company product group to make a market entry at the earliest
possible time; they have also requested a complete project plan up to the startup of production.
Project responsibility is assigned to the division’s research and development group.
Sarah Morrison, the project scientist who developed the product, is assigned responsibility for
project management. Assistance will be required from other parts of the company: Design
Engineers, R&D group, Manufacturing personnel, and Supply Chain group.
Sarah was concerned about the scope of the project. She knew from her own experience
that a final formula had yet to be developed, although such development was really a “routine”
task. The remaining questions had to do with color, odor, and consistency additives rather than
any performance-related modification. Fortunately, the major regulatory issues had also been
resolved and he believed that submission of regulatory documentation would be followed by
rapid approval as they already had a letter of approval contingent on final documentation.
Sarah was concerned about defining the project unambiguously. To that end, She
obtained an interview with William Richardson, the group vice-president. When She asked Mr.
Richardson where his responsibility should end, the executive turned the question back to him.
Sarah Morrison had been prepared for this and said that she would like to regard her part of the
project as done when the production process could be turned over to manufacturing. They
agreed that according to Triad’s practice, this would be when the manufacturing operation
could produce a 95.5% yield of product (fully packaged) at a level of 97% of the full
production goal of 16 million liters per year.
“But I want you to remember,” said Mr. Richardson, “that you must meet all current
FDA, EPA, and OSHA regulations and you must be in compliance with our internal
specification - the one I have is dated September and is RD15/92020X. And you know that
manufacturing now - quite rightly, I feel - insists on fully documented procedures.”
After this discussion, Sarah felt that She had enough information about this aspect to
start to pin down what had to be done to achieve these results. Her first step in this effort was
to meet with Karen Johnson, the director of research.
“You are naïve if you think that you can just start right in finalizing the formula,” said
Ms. Johnson. You must first develop a product rationale (a). This is a formally defined process
according to company policy. Marketing expects inputs at this stage, manufacturing expects its
voice heard, and you will have to have approvals from every unit of the company that is
involved. You should have no trouble if you do your homework, expect to spend a good eight
weeks to get this (i.e., project rationale) done. “Do you remember the project concept
definition from your project management class”, she quipped?
“That certainly stretches things out,” said Sarah. “I expect to take 12 weeks to develop
the ingredient formula (b) and you know that I can’t start to establish product specifications (c)
until the formula is complete. That’s another 3 weeks.”
“Yes, but while you are working on the product specifications you can get going on the
regulatory documentation (d). Full internal specifications are not required for that work, but
you can’t start those documents until the formula is complete.” “Yes, and I find it hard to
2
believe that we can push through both preparation of documents and getting approval in 3
weeks, but Environmental swears it can be done.”
“Oh, it can be done in this case because of the preparatory work. Of course, I won’t say
that this estimate of 3 weeks is as certain as our other estimates. All we need is a change of
staff at the Agency and we are in trouble. But once you have both the specifications, and the
approval done, you can immediately start on developing the processing system (g).”
"Yes, and how I wish we could get a lead on that, but the designers say that there is too
much uncertainty and they won't move until they have both specifications and regulatory
documentation and approval. They are offering pretty fast response; six weeks from start to
finish for the processing system."
"They are a good crew, Sarah. And of course, you know that you don't have to delay
on starting the packaging segment of this project. You can start developing the packaging
concept (e) just as soon as the product rationale has been developed. If my experience is any
judge, it will take a full eight weeks; you'll have to work to keep the process from running
forever."
"But as soon as that is finished we can start on the design of the package and its
materials (f) which usually takes about six weeks. Once that is done, we can start on the
packaging system (h) which shouldn't take longer than eight weeks," concluded Sarah
Morrison. At this point she realized that although Karen Johnson would have general
knowledge, She needed to talk directly to the Director of Manufacturing.
"The first step, which follows the completion of the development of processing and
packaging systems," said the Director of Manufacturing, "is to do a complete study of the
facilities requirements (i). You won't be able to get that done in less than four weeks. And that
must precede the preparation of the capital equipment list (j) which should take about three-
quarters as long. Of course, as soon as both the process system and packaging system are
completed, you could start on preparing the written manufacturing procedures (q)."
"But, said Sarah, "Can I really finish the procedures before I have installed and
constructed the facilities (p)?"
"No, quite right. What you can do is get the first phase of the written procedures done,
but the second phase will have to wait for the installation and construction of facilities."
"Then this means that I really have two phases for the writing of the procedures, that
which can be completed without the installation and construction (q1), which will take seven
weeks, and that which has to wait for those inputs (q2) which will require 3 weeks."
"True. Now you realize that the last thing you have to do is to run the equipment in a
pilot test (r) which will show that you have reached a satisfactory level of yield and volume?"
"Yes. Since that must include debugging, I've estimated a six-week period as
adequate." The director of manufacturing assented. Sarah continued, "What I'm not sure of is
whether we can run all the installation tasks in parallel."
"You can get the purchase orders and carry out the procurement of process equipment
(k), packaging equipment (l), and facilities (m) as soon as the capital equipment list is
complete. The installation of each of these types of equipment and facilities can start as soon
as the specific subsystem is on hand (n,o,p)".
"What do you estimate for the times to do these tasks?" asked Sarah. The Director of
Manufacturing estimated 18, 8, and 4 weeks for the purchasing phases for each of the
subsystems in that order and four weeks for each of the installations. "Then I can regard my
job as done with the delivery of the procedures and when I show my 95.5 percent yield," said
3
Sarah Morrison, and the Director of Manufacturing agreed, but reminded Sarah that none of
the purchasing cycles could start until the capital equipment list had been prepared and
approved (j) which she saw as a three-week task.
The executive committee of Triad Pharmaceuticals set a starting date for the project of
May 3, 2021 and asked Sarah to project a completion date with his submission of the plan.
The committee's request implied that whatever date Sarah came up with was acceptable, but
Sarah knew that she would be expected to show how to shorten the time to complete the
project. However, her task in making the schedule was clear; she had to establish the resource
requirements and deal with calendar constraints as best as she could.
To this end, Sarah had to get an estimate of types resources which she decided to do by
making a list of the activities and asking each group involved what was their level of employee
input. The results of this survey are shown in Exhibit 1.
Sarah knew that it was customary at Traid Pharmaceuticals to provide the following as
parts of a project plan to be submitted to the executive committee:
1. The project’s goals and objectives (i.e. when will Sarah’s work be done, and not your
class project). Keep in mind a project’s objectives include identification of the project’s
mission. (8 pts)
2. A work breakdown structure (an organization chart type preferred) (8 pts)
3. A fully labeled network diagram (PERT Chart), Format your chart that it will fit on one
page if it were to be printed. It must be clearly readable. (6 pts)
4. A determination of the critical path or paths and the original expected duration of the
project (provide your answer in weeks). Write this out completely in your report (6 pts)
5. An informative Early-Start Schedule (a Gantt Chart), in which every activity would be
started at its Early Start time, regardless of resource constraints. Format your Gantt
chart that it will fit on one page if it were to be printed. It must be clearly readable. (6
pts)
6. A resource loading graph showing the period by period resource requirements for each
group of workers (one graph for Design Engineer, one graph for R&D Group, etc).
Each resource graph should fit on one page if it were to be printed. (10 pts)
7. A comparative analysis showing the tradeoffs between the extra workers needed to
remove any labor over-allocations; and the minimum time the project could be
completed if extra resources are not available.
That is,
i. provide an analysis that shows the minimum number of workers in each group
needed if the original deadline is to be maintained.
ii. Provide another analysis that shows how long it will take to complete the
project if there are no additional resources beyond what is currently provided.
4
iii. Provide a third analysis that shows some middle ground between those two
extremes (I & II above). Attach a Gantt chart for each of your three scenarios.
(10 pts)
8. An identification of any potential problems and risks with the entire plan (not just the
resources) that you have developed and any opportunities within the plan (problems
unique to this project) for addressing those problems. (6 pts)
Submit a report to top management with an executive summary of your project plan addressing
all 8 items listed above. The report should reflect high professionalism. Each diagram must be
inserted into its appropriate place within the report or attached as an appendix and must be
readable.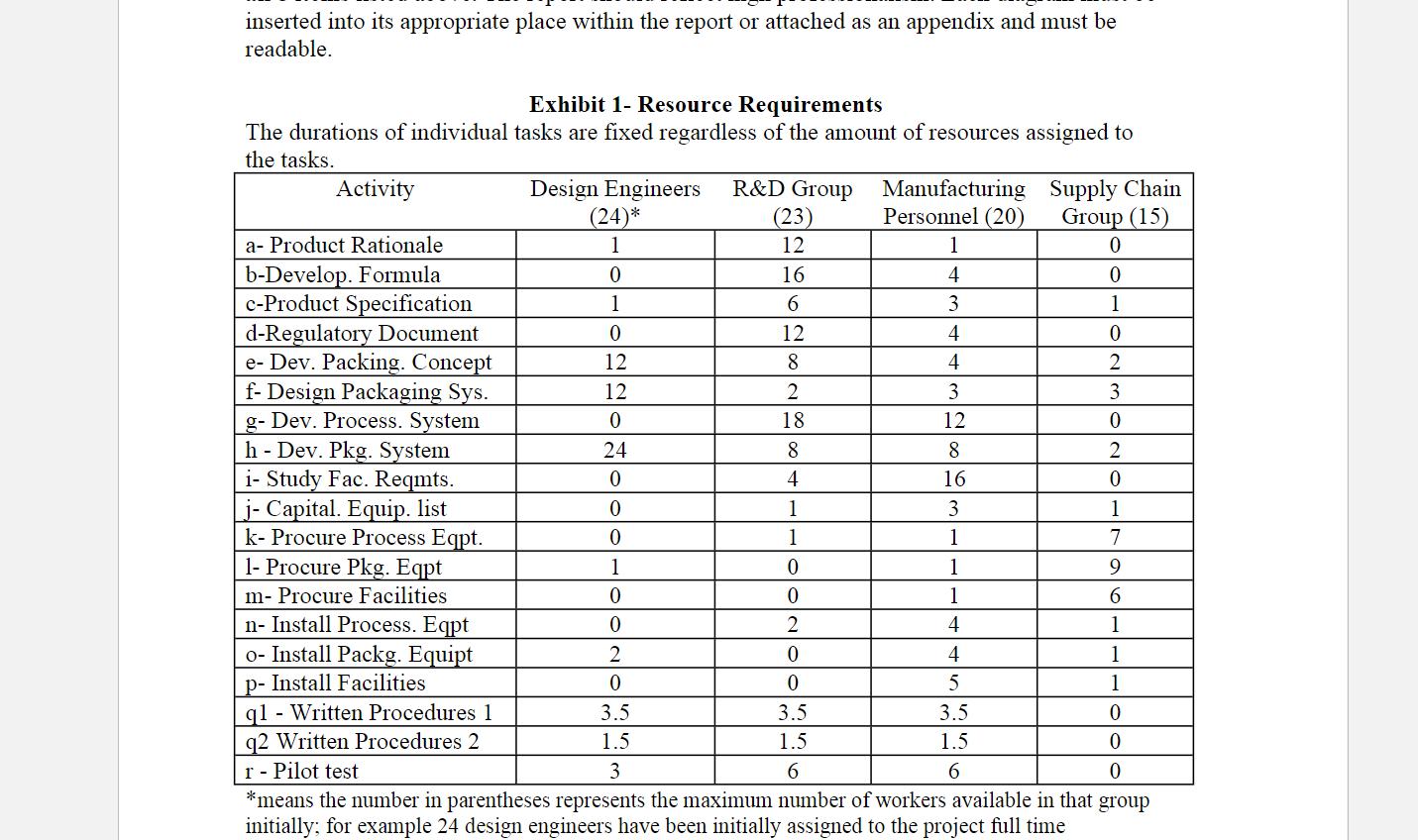
inserted into its appropriate place within the report or attached as an appendix and must be readable. Exhibit 1- Resource Requirements The durations of individual tasks are fixed regardless of the amount of resources assigned to the tasks. Activity a- Product Rationale b-Develop. Formula c-Product Specification d-Regulatory Document e- Dev. Packing. Concept f- Design Packaging Sys. g- Dev. Process. System h - Dev. Pkg. System i- Study Fac. Reqmts. j- Capital. Equip. list k- Procure Process Eqpt. 1- Procure Pkg. Eqpt m- Procure Facilities n- Install Process. Eqpt Design Engineers R&D Group Manufacturing Supply Chain Group (15) Personnel (20) 0 0 1 0 2 o- Install Packg. Equipt p- Install Facilities q1 - Written Procedures 1 q2 Written Procedures 2 r - Pilot test (24)* 1 0 1 0 12 12 0 24 0 (23) 12 16 6 0 0 1 0 0 2 0 3.5 1.5 3 12 8 2 18 8 4 1 1 0 0 2 1 7 9 6 1 1 1 0 0 0 *means the number in parentheses represents the maximum number of workers available in that group initially; for example 24 design engineers have been initially assigned to the project full time 0 0 1 4 3 4 4 3 12 8 16 3 1 1 1 4 4 5 3.5 1.5 6 3.5 1.5 6 3 0 2 0
Step by Step Solution
3.37 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Answer The activity definition method may be a any breakdown of the work package components of the WBS It documents the precise activities required to meet the deliverables elaborated within the WBS T...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


