DETERMINATION OF IRON CONTENT IN A FERROUS AMMONIUM SULFATE UNKNOWN Obtain an unknown sample from your professor and record the unknown number in your

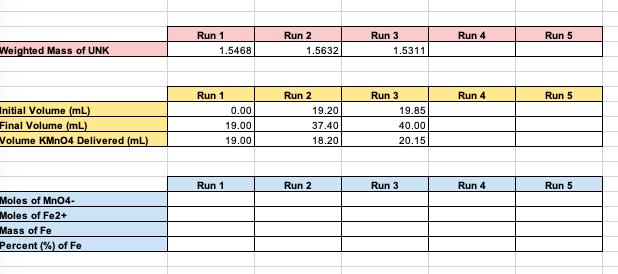
DETERMINATION OF IRON CONTENT IN A FERROUS AMMONIUM SULFATE UNKNOWN Obtain an unknown sample from your professor and record the unknown number in your notebook. Weigh out 3-4 samples of between 1.5000g and 2.0000g ferrous ammonium sulfate unknown and place in 250 mL flasks. Add 15 mL of distilled water and 1 mL of concentrated HCI to each sample. Swirl to dissolve sample completely. Gently warm on a hot plate at your bench. Wash down the sides of the flask with some distilled water. Add 0.2 M Potassium Permanganate solution (not your standardized solution) dropwise until a distinct yellow color forms. (should be about 2 drops) Carry out the following procedure individually on each sample. Add 25 mL of the Zimmermann-Reinhardt reagent and 300 mL of distilled water. Titrate immediately with your standardized KMnO4 solution to the first faint pink color that persists for 15 to 30 seconds. Do not titrate rapidly at any time. The pink color results from excess MnO4, indicating that all Fe+ has been oxidized to Fe+, Weighted Mass of UNK Initial Volume (mL) Final Volume (mL) Volume KMnO4 Delivered (mL) Moles of MnO4- Moles of Fe2+ Mass of Fe Percent (%) of Fe Run 1 1.5468 0.00 19.00 19.00 Run 1 Run 1 Run 2 1.5632 19.20 37.40 18.20 Run 2 Run 2 Run 3 1.5311 19.85 40.00 20.15 Run 3 Run 3 Run 4 Run 4 Run 4 Run 5 Run 5 Run 5
Step by Step Solution
3.50 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Molasity of Initial V ml 010 1920 1985 Final V ml 190 3740 4000 V 190 ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


