Answered step by step
Verified Expert Solution
Question
1 Approved Answer
determine its reaction order (show in y=mx+b) and its k constant. calculate the (OH-) Time Absorbance Concentration of In (R)(M) 1/ (+)(10*M1) (Minutes) Crystal Violet,
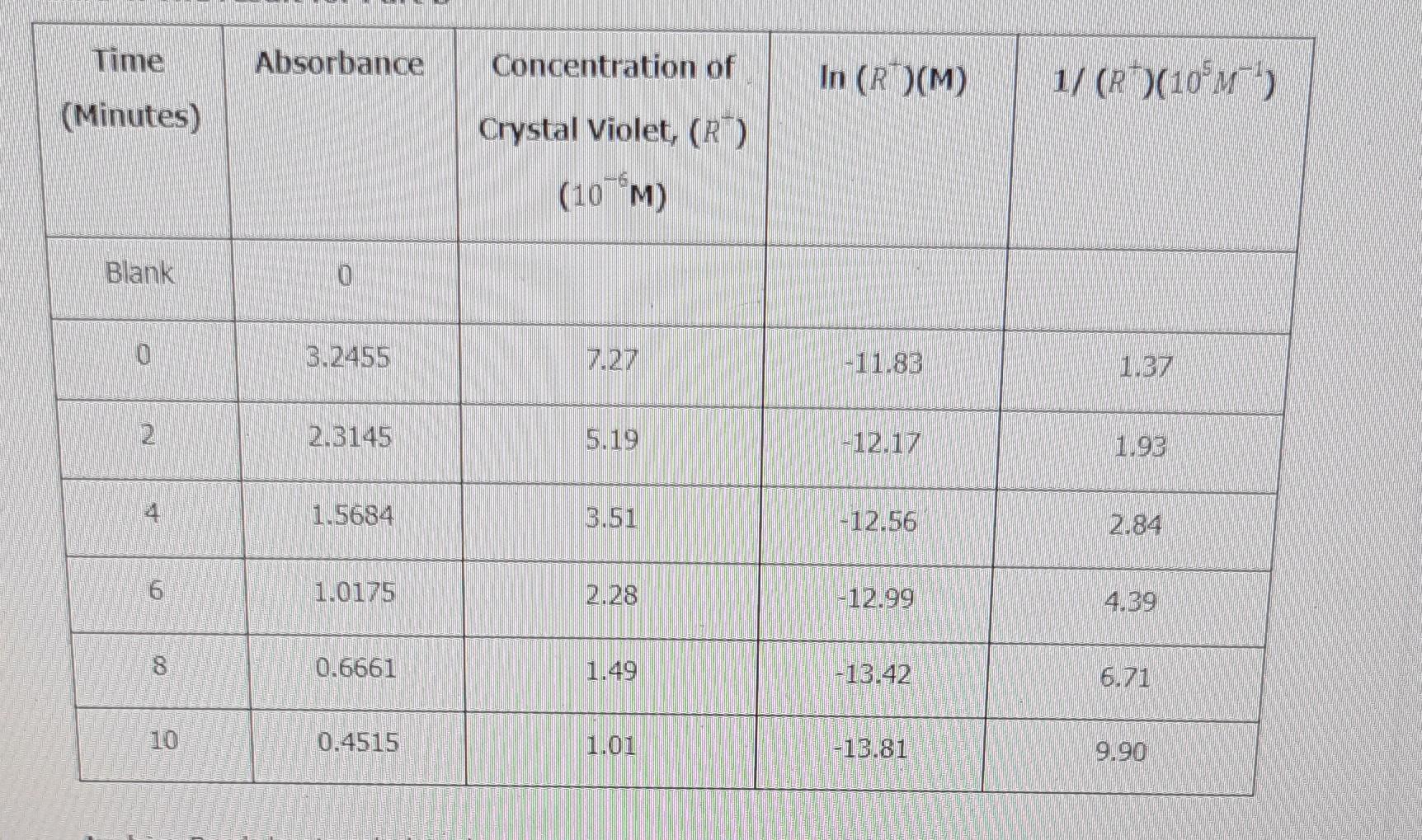
determine its reaction order (show in y=mx+b) and its k constant. calculate the (OH-)
Time Absorbance Concentration of In (R)(M) 1/ (+)(10*M1) (Minutes) Crystal Violet, (R) (107M) Blank 0 0 3.2455 7.27 -11.83 1.37 2 2.3145 5.19 -12.17 1.93 1.5684 3.51 -12.56 2.84 6 1.0175 2.28 -12.99 4.39 8 0.6661 1.49 -13.42 6.71 10 0.4515 1.01 -13.81 9.90Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


