Question
1. The following table gives common radioisotopes in nuclear medicine. Isotope Half-life 13.2 hours 60.14 days 1231 (Iodine 123) 1251 (lodine 125) 1311 (lodine
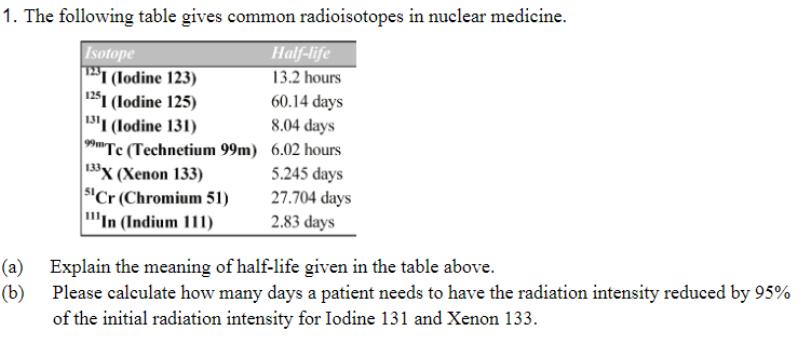
1. The following table gives common radioisotopes in nuclear medicine. Isotope Half-life 13.2 hours 60.14 days 1231 (Iodine 123) 1251 (lodine 125) 1311 (lodine 131) 8.04 days 99mTe (Technetium 99m) 6.02 hours 133X (Xenon 133) 5.245 days "Cr (Chromium 51) 27.704 days 'In (Indium 111) 2.83 days (a) Explain the meaning of half-life given in the table above. (b) Please calculate how many days a patient needs to have the radiation intensity reduced by 95% of the initial radiation intensity for Iodine 131 and Xenon 133.
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
a The halflife of a radioisotope is the time taken for half of the radioactive nuclei in a sample to ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



