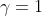Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determine the percentage of liquid and vapor formed when a mixture containing the following mole fractions 0.1835 N 2 , 0.5506 H 2 , 0.2658
Determine the percentage of liquid and vapor formed when a mixture containing the following mole fractions 0.1835 N2, 0.5506 H2, 0.2658 NH3 enters a flash separator at -25C and 20 bar. Data provided:
[1] N2
[2] H2
[3] NH3
Components at the entrance:
Z1 = 0.18
Z2 = 0.54
Z3 = 0.28
Input current:
F = 2439.8
L = 2439.8 - V
Distribution coefficients K:
K1 = 1300
K2 = 2400
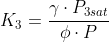
P3sat = 1.51779 bar
*For the calculation of the fugacity, ideality can be assumed because pure ammonia is obtained. An activity of 1 is assumed*
The following equation can be used if necessary:
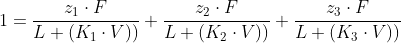
K3 = 7. P3sat 0.P P =4 7= 21F 2.F 22 23F 1 = L+(K1.V)) L +(K2V)) L +(K3.V))
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started