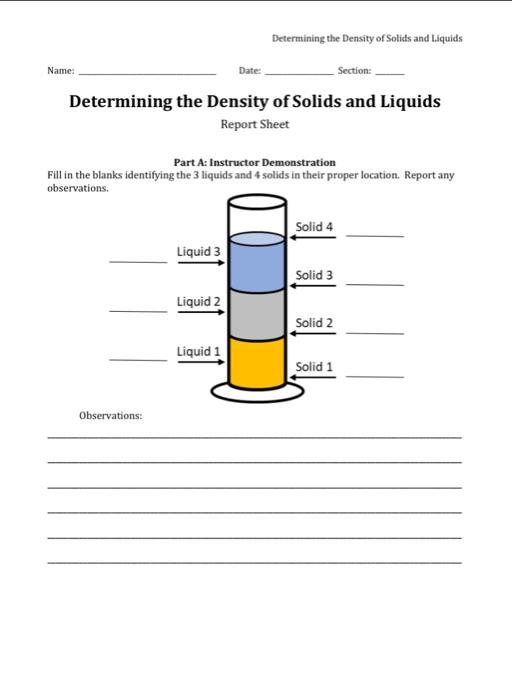
Determining the Density of Solids and Liquids Objectives 1. Utilize a variety of measuring devices to determine mass and volume to the proper number of significant figures (digits) for regular-and irregular-shaped solids, as well as liquids. 2. Calculate the densities of the different substances and the thickness of foil. Introduction In this lab, you will be introduced to both intensive and extensive properties. Extensive properties depend on the amount (extent) of material involved, whereas intensive properties do not depend on the amount of material present. Density (D), an intensive property, is defined as mass per unit volume (mass/volume); obviously, mass and volume are extensive properties: D=Vm For most solids and liquids, the mass is measured in grams, and the volume is measured either in cubic centimeters or in milliliters, resulting in density having units of either g/cm1 or g/mL since 1 cm3=1mL. A density determination involves weighing a measured volume of a substance. An object's mass can be measured on a weighing balance. When determining the mass of a liquid, the indirect technique, weighing by difference, must be used. This is done by weighing an empty container, then adding a specific volume of liquid, reweighing the container, and subtracting the masses to determine the mass of the liauld. Figure 1: Weighing by difference: The mass of the H2O can be determined by taking the difference in masses: 35.522g25.412g=10.110gH20 The volume of a solid can be determined directly only if the solid has a regular geometry (e.g a regular cone, a cube, a cylinder, a rectangular solid or a sphere) by simply measuring its dimensions and using a formula to calculate the volume. When, however, the solid is irregular in shape, a convenient substitution for this method is to use a technique called volume by displacement, to measure the volume displaced by the solid when submerged in a liquid. When submerged, the solid will displace a volume of the liquid equal to the volume of the solid. The volume of the solid, then, can be calculated by taking the volume of the liquid with the solid submerged minus the volume of the liquid without the solid submerged. For this method to work well, the solid must not react with, or dissolve in, the liquid chosen for the determination. Preferably, the liquid should also have a lower density than the solid (otherwise, the solid would have to be held under the surface to be completely submerged). For liquids, the volume can be measured simply using a volumetric device such as a graduated cylinder. Determining the Density of Solids and Lquids Figure 2: Volume by Displacement: The volume of the irregular shaped object can be determined by taking the difference in volume: 29.5mL20.9mL=8.6mL During this experiment you will be using several different measuring devices for determining densities. In order to make proper measurements, you must record each quantity with the appropriate number of significant digits based on the device's precision (the first significant figure is the first nonzero digit in your measurement and the last significant figure is the digit at the end of your measurement, the one that is estimated (often by interpolating between two calibration marks). The more significant digits you have, the more precise (not necessarily more accurate) the measurement. In this experiment, you will also be asked to determine the thickness of aluminum foil since it is too thin to actually measure. By using the density of foil, we can calculate the volume of a piece of foil and then calculate the thickness. Figure 3: Thickness of foik the thickness of foil can be calculated by measuring the length, width and mass of a piece of foil. Density of aluminum foil =2.70g/cm3. V=Dm=2.70cm3E0.315F=0.11667cm3V=1wtt=1wV=9.48cm5.04cm0.11667cm3=0.0024419cm Report the final answer using proper significant figures: 0.00244cm or 2.44101cm. ExperimentalProcedure Do NOT take water bottles into the balance room! Part A:Instructor Demonstration: 1. Your lab instructor will place three chemicals in a graduated cylinder which will form three layers. Record which chemical is in each layer. 2. Record observation of location when a glass marble is placed in the graduated cylinder. 3. Record observation of location when a piece of red hose is placed in the graduated cylinder. 4. Record observation of location when a rubber stopper is placed in the graduated cylinder. 5. Record observation of location when a cork is placed in the graduated cylinder. Part B:Density of Water 1. Mass: Determine the mass of a clean, dry 50mL beaker. 2. Volume: Using your pipet, deliver a 10.00-mL sample of water into the beaker. Be aware of what kind of pipet used: "to deliver" or "to contain". You want the "to deliver" kind. Note BE SURE TO KEEP LIQUIDS OUT OF THE SUCTION DEVICE! 3. Re-weigh the beaker (with the liquid). 4. Determine the mass of liquid delivered by the pipet by using the following formula: mass of liquid = (mass of beaker and liquid) mass of beaker 5. Repeat steps 1-3 a second time, using a clean, beaker. 6. Density; Calculate two densities of the liquid from the two dividing each mass in grams by 10.00-mL 7. Mean: Calculate the mean density. 4. Yolume: Put 3560mL of water into a 100-mL graduated cylinder. Record the volume to the nearest 0.1mL. Gently, place the rubber stopper into the graduated cylinder and record the new volume (to the nearest 0.1mL.). Calculate the volume of the substance by the difference in the volumes. 5. Density: Calculate the density of the rubber stopper using your data from above. Repeat the procedure for an additional trial with different rubher stonper. 6. Mean: Calculate the mean density. Part D: Density of a Rectangular Block: 1. Mass: Obtain a block and determine the mass of the object, recording the mass with as many significant figures (digits) as the balance will allow. 2. Volume: Accurately measure the length (I), width ( w ), and height ( h ) of the rectangular solid. Use the maximum precision of the ruler (nearest 0.01cm ). Determine the volume as seen in under Sample Calculations Part 1C. 3. Density: Determine the density of the block. Ensure that you report your answer with the correct number of significant digits! Rart E: Thickness of Aluminum Foil: 1. Determine the length and width of a rectangular piece of aluminum foil. Use the maximum precision of the ruler (nearest 0.01cm ). 2. Mass: Fold the foil 3-4 times. Weigh and record the mass with as many significant figures (digits) as the balance will allow. 3. Using density of foil =2.70g/cm3, find the volume of the foil. Then find the thickness of the foil. Waste.Disposal 1. All liquids are to be flushed down the sink with water. C. In Part D, the width, length and height of a rectangular block are found. The volume is found using the simple equation: V=Iwh. For example, if a student finds the length of the sides to be 2.54cm,3.64cm, and 5.48cm, V=2.54cm3.64cm5.48cm=50.6cm3 D. In Part E, the thickness of foil is to be calculated. First, the volume will need to be determined using the mass and given density {2.70g/cm2). If foil =0.415R, then Sincedensity=volumemassthenvolume=densitymassVolume=2.70cm3g0.415g=0.154cm3 The thickness can now be determined using the calculated volume and the measured length and width. If length =3.25cm and width =3.45cm, then SinceV=1wtthent=V/(1w)t=0.154cm2/(3.25cm3.45cm)=t=0.154cm2/(11.2125cm2)=0.0137cm How do 1 find density? A. Once the volume and the mass are known, they can simply be substituted into the density equation. For example, using the volume found in example 1C (above) and a mass of 60.451g the calculation is shown below. Density=volumemass=50.6rm260.451g=1.19cm2g B. Calculating a mean. Suppose your densities were 1.2, 1.5, and 1.8g/ml, respectively. x=31.2g/mL+1.5g/ml+1.8g/ml=1.5mLg Determining the Density of Solids and Liquids Report Sheet Part A: Instructer Demonstration Fill in the blanks identifying the 3 liquids and 4 solids in their proper location. Report any observatione Determining the Density of Solids and Liquids F Determining the Density of Solids and Liquids Discussion Questions 1. Based on your calculated density of the block, would it float or sink in water? Explain. 2. Based on your average calculated density of the water, do you think it is accurate? Explain. (True density of water is 0.998203g/mL ) Determining the Density of Solids and Liquids Objectives 1. Utilize a variety of measuring devices to determine mass and volume to the proper number of significant figures (digits) for regular-and irregular-shaped solids, as well as liquids. 2. Calculate the densities of the different substances and the thickness of foil. Introduction In this lab, you will be introduced to both intensive and extensive properties. Extensive properties depend on the amount (extent) of material involved, whereas intensive properties do not depend on the amount of material present. Density (D), an intensive property, is defined as mass per unit volume (mass/volume); obviously, mass and volume are extensive properties: D=Vm For most solids and liquids, the mass is measured in grams, and the volume is measured either in cubic centimeters or in milliliters, resulting in density having units of either g/cm1 or g/mL since 1 cm3=1mL. A density determination involves weighing a measured volume of a substance. An object's mass can be measured on a weighing balance. When determining the mass of a liquid, the indirect technique, weighing by difference, must be used. This is done by weighing an empty container, then adding a specific volume of liquid, reweighing the container, and subtracting the masses to determine the mass of the liauld. Figure 1: Weighing by difference: The mass of the H2O can be determined by taking the difference in masses: 35.522g25.412g=10.110gH20 The volume of a solid can be determined directly only if the solid has a regular geometry (e.g a regular cone, a cube, a cylinder, a rectangular solid or a sphere) by simply measuring its dimensions and using a formula to calculate the volume. When, however, the solid is irregular in shape, a convenient substitution for this method is to use a technique called volume by displacement, to measure the volume displaced by the solid when submerged in a liquid. When submerged, the solid will displace a volume of the liquid equal to the volume of the solid. The volume of the solid, then, can be calculated by taking the volume of the liquid with the solid submerged minus the volume of the liquid without the solid submerged. For this method to work well, the solid must not react with, or dissolve in, the liquid chosen for the determination. Preferably, the liquid should also have a lower density than the solid (otherwise, the solid would have to be held under the surface to be completely submerged). For liquids, the volume can be measured simply using a volumetric device such as a graduated cylinder. Determining the Density of Solids and Lquids Figure 2: Volume by Displacement: The volume of the irregular shaped object can be determined by taking the difference in volume: 29.5mL20.9mL=8.6mL During this experiment you will be using several different measuring devices for determining densities. In order to make proper measurements, you must record each quantity with the appropriate number of significant digits based on the device's precision (the first significant figure is the first nonzero digit in your measurement and the last significant figure is the digit at the end of your measurement, the one that is estimated (often by interpolating between two calibration marks). The more significant digits you have, the more precise (not necessarily more accurate) the measurement. In this experiment, you will also be asked to determine the thickness of aluminum foil since it is too thin to actually measure. By using the density of foil, we can calculate the volume of a piece of foil and then calculate the thickness. Figure 3: Thickness of foik the thickness of foil can be calculated by measuring the length, width and mass of a piece of foil. Density of aluminum foil =2.70g/cm3. V=Dm=2.70cm3E0.315F=0.11667cm3V=1wtt=1wV=9.48cm5.04cm0.11667cm3=0.0024419cm Report the final answer using proper significant figures: 0.00244cm or 2.44101cm. ExperimentalProcedure Do NOT take water bottles into the balance room! Part A:Instructor Demonstration: 1. Your lab instructor will place three chemicals in a graduated cylinder which will form three layers. Record which chemical is in each layer. 2. Record observation of location when a glass marble is placed in the graduated cylinder. 3. Record observation of location when a piece of red hose is placed in the graduated cylinder. 4. Record observation of location when a rubber stopper is placed in the graduated cylinder. 5. Record observation of location when a cork is placed in the graduated cylinder. Part B:Density of Water 1. Mass: Determine the mass of a clean, dry 50mL beaker. 2. Volume: Using your pipet, deliver a 10.00-mL sample of water into the beaker. Be aware of what kind of pipet used: "to deliver" or "to contain". You want the "to deliver" kind. Note BE SURE TO KEEP LIQUIDS OUT OF THE SUCTION DEVICE! 3. Re-weigh the beaker (with the liquid). 4. Determine the mass of liquid delivered by the pipet by using the following formula: mass of liquid = (mass of beaker and liquid) mass of beaker 5. Repeat steps 1-3 a second time, using a clean, beaker. 6. Density; Calculate two densities of the liquid from the two dividing each mass in grams by 10.00-mL 7. Mean: Calculate the mean density. 4. Yolume: Put 3560mL of water into a 100-mL graduated cylinder. Record the volume to the nearest 0.1mL. Gently, place the rubber stopper into the graduated cylinder and record the new volume (to the nearest 0.1mL.). Calculate the volume of the substance by the difference in the volumes. 5. Density: Calculate the density of the rubber stopper using your data from above. Repeat the procedure for an additional trial with different rubher stonper. 6. Mean: Calculate the mean density. Part D: Density of a Rectangular Block: 1. Mass: Obtain a block and determine the mass of the object, recording the mass with as many significant figures (digits) as the balance will allow. 2. Volume: Accurately measure the length (I), width ( w ), and height ( h ) of the rectangular solid. Use the maximum precision of the ruler (nearest 0.01cm ). Determine the volume as seen in under Sample Calculations Part 1C. 3. Density: Determine the density of the block. Ensure that you report your answer with the correct number of significant digits! Rart E: Thickness of Aluminum Foil: 1. Determine the length and width of a rectangular piece of aluminum foil. Use the maximum precision of the ruler (nearest 0.01cm ). 2. Mass: Fold the foil 3-4 times. Weigh and record the mass with as many significant figures (digits) as the balance will allow. 3. Using density of foil =2.70g/cm3, find the volume of the foil. Then find the thickness of the foil. Waste.Disposal 1. All liquids are to be flushed down the sink with water. C. In Part D, the width, length and height of a rectangular block are found. The volume is found using the simple equation: V=Iwh. For example, if a student finds the length of the sides to be 2.54cm,3.64cm, and 5.48cm, V=2.54cm3.64cm5.48cm=50.6cm3 D. In Part E, the thickness of foil is to be calculated. First, the volume will need to be determined using the mass and given density {2.70g/cm2). If foil =0.415R, then Sincedensity=volumemassthenvolume=densitymassVolume=2.70cm3g0.415g=0.154cm3 The thickness can now be determined using the calculated volume and the measured length and width. If length =3.25cm and width =3.45cm, then SinceV=1wtthent=V/(1w)t=0.154cm2/(3.25cm3.45cm)=t=0.154cm2/(11.2125cm2)=0.0137cm How do 1 find density? A. Once the volume and the mass are known, they can simply be substituted into the density equation. For example, using the volume found in example 1C (above) and a mass of 60.451g the calculation is shown below. Density=volumemass=50.6rm260.451g=1.19cm2g B. Calculating a mean. Suppose your densities were 1.2, 1.5, and 1.8g/ml, respectively. x=31.2g/mL+1.5g/ml+1.8g/ml=1.5mLg Determining the Density of Solids and Liquids Report Sheet Part A: Instructer Demonstration Fill in the blanks identifying the 3 liquids and 4 solids in their proper location. Report any observatione Determining the Density of Solids and Liquids F Determining the Density of Solids and Liquids Discussion Questions 1. Based on your calculated density of the block, would it float or sink in water? Explain. 2. Based on your average calculated density of the water, do you think it is accurate? Explain. (True density of water is 0.998203g/mL )