Answered step by step
Verified Expert Solution
Question
1 Approved Answer
di- tert -butyl peroxide, (CH3)3COOC(CH3)3 decomposes into 2(CH3)2CO and C2H6 The reaction takes place in a reactor of 400mL volume at 150 C. All species
di-tert-butyl peroxide, (CH3)3COOC(CH3)3 decomposes into 2(CH3)2CO and C2H6
The reaction takes place in a reactor of 400mL volume at 150 C. All species are in the gaseous state. The gas phase consists initially of nitrogen with a partial pressure of 5 mmHg and di-tert -butyl peroxide with a partial pressure of 170 mmHg. The following table gives the total pressure measured at consecutive times.
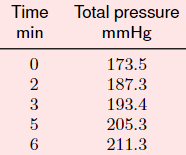
Calculate the extent of reaction and the extent of reaction density.
Time Total pressure min mmHg 0 173.5 2 187.3 3 193.4 5 205.3 6 211.3Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


