Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Dilute Hydroiodic Acid (HI) is a pharmaceutical product containing 10% HI and about 0.8% hypophosphorous acid (H3PO2), to prevent discoloration of the aqueous preparation
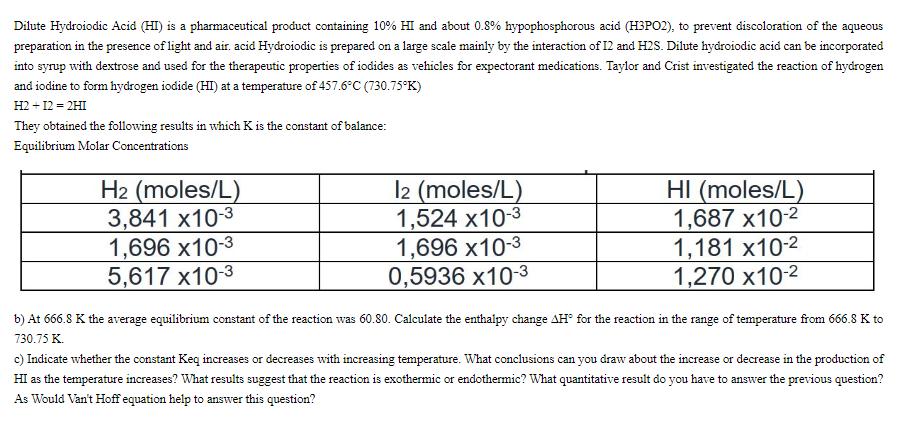
Dilute Hydroiodic Acid (HI) is a pharmaceutical product containing 10% HI and about 0.8% hypophosphorous acid (H3PO2), to prevent discoloration of the aqueous preparation in the presence of light and air. acid Hydroiodic is prepared on a large scale mainly by the interaction of 12 and H2S. Dilute hydroiodic acid can be incorporated into syrup with dextrose and used for the therapeutic properties of iodides as vehicles for expectorant medications. Taylor and Crist investigated the reaction of hydrogen and iodine to form hydrogen iodide (HI) at a temperature of 457.6C (730.75K) H2 + 12 = 2HI They obtained the following results in which K is the constant of balance: Equilibrium Molar Concentrations H2 (moles/L) 3,841 x10-3 1,696 X10-3 5,617 X10-3 12 (moles/L) 1,524 x10-3 1,696 X10-3 0,5936 X10-3 HI (moles/L) 1,687 X10- 1,181 x10- 1,270 x10- b) At 666.8 K the average equilibrium constant of the reaction was 60.80. Calculate the enthalpy change AH for the reaction in the range of temperature from 666.8 K to 730.75 K. c) Indicate whether the constant Keq increases or decreases with increasing temperature. What conclusions can you draw about the increase or decrease in the production of HI as the temperature increases? What results suggest that the reaction is exothermic or endothermic? What quantitative result do you have to answer the previous question? As Would Van't Hoff equation help to answer this question?
Step by Step Solution
★★★★★
3.52 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


