Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Dipole moment, (Hexane,Cyclohexane,Toluene) and refractive index, The data are as follows: Flask 1 2 3 20 18 1.89 2.015 18.5 0.9484 2.379 The factor
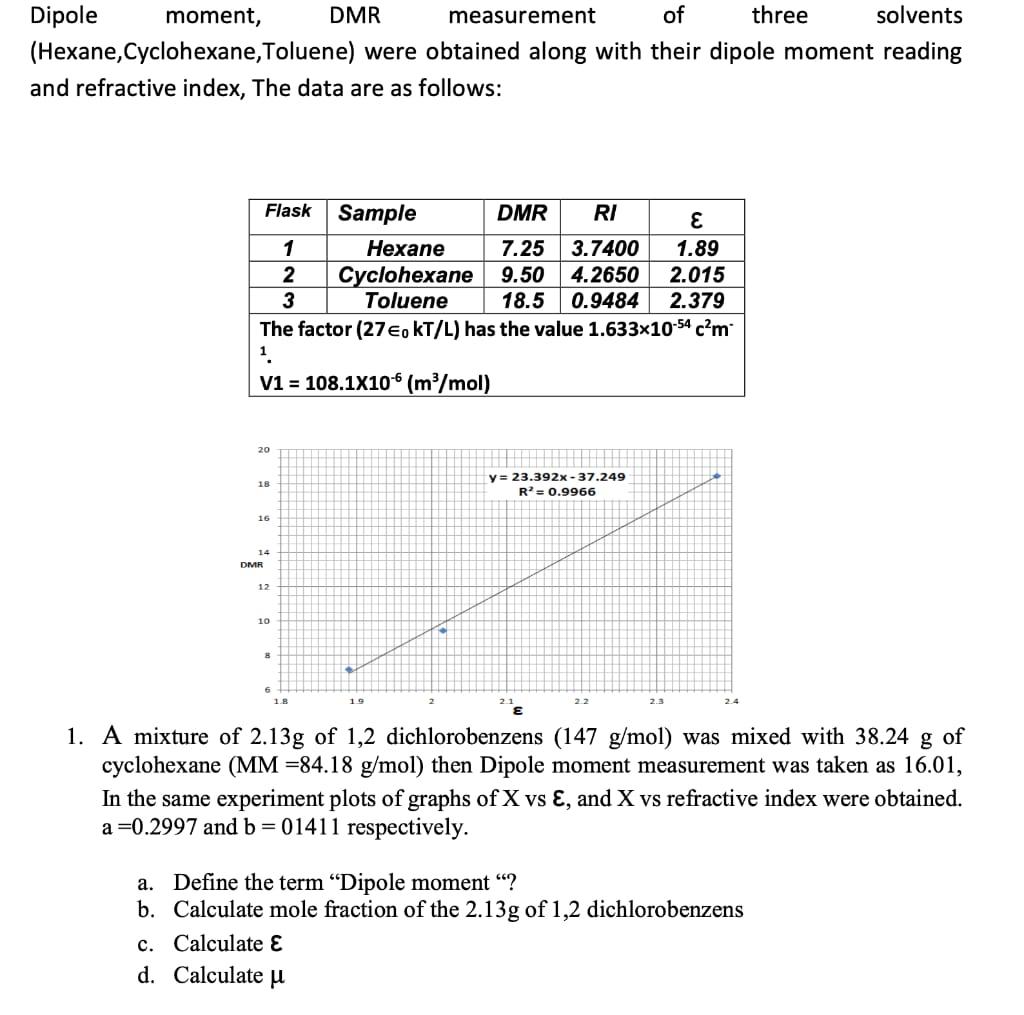
Dipole moment, (Hexane,Cyclohexane,Toluene) and refractive index, The data are as follows: Flask 1 2 3 20 18 1.89 2.015 18.5 0.9484 2.379 The factor (27, KT/L) has the value 1.633x10-54 cm 1. V1 = 108.1X106 (m/mol) 16 14 DMR 12 10 8 6 DMR measurement of three solvents were obtained along with their dipole moment reading 1.8 DMR RI Hexane 7.25 3.7400 Cyclohexane 9.50 4.2650 Toluene Sample c. Calculate E d. Calculate 19 y= 23.392x-37.249 R = 0.9966 2.2 2.3 1. A mixture of 2.13g of 1,2 dichlorobenzens (147 g/mol) was mixed with 38.24 g of cyclohexane (MM -84.18 g/mol) then Dipole moment measurement was taken as 16.01, In the same experiment plots of graphs of X vs E, and X vs refractive index were obtained. a=0.2997 and b = 01411 respectively. a. Define the term "Dipole moment "? b. Calculate mole fraction of the 2.13g of 1,2 dichlorobenzens Dipole moment, (Hexane,Cyclohexane,Toluene) and refractive index, The data are as follows: Flask 1 2 3 20 18 1.89 2.015 18.5 0.9484 2.379 The factor (27, KT/L) has the value 1.633x10-54 cm 1. V1 = 108.1X106 (m/mol) 16 14 DMR 12 10 8 6 DMR measurement of three solvents were obtained along with their dipole moment reading 1.8 DMR RI Hexane 7.25 3.7400 Cyclohexane 9.50 4.2650 Toluene Sample c. Calculate E d. Calculate 19 y= 23.392x-37.249 R = 0.9966 2.2 2.3 1. A mixture of 2.13g of 1,2 dichlorobenzens (147 g/mol) was mixed with 38.24 g of cyclohexane (MM -84.18 g/mol) then Dipole moment measurement was taken as 16.01, In the same experiment plots of graphs of X vs E, and X vs refractive index were obtained. a=0.2997 and b = 01411 respectively. a. Define the term "Dipole moment "? b. Calculate mole fraction of the 2.13g of 1,2 dichlorobenzens
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Dipole moment occur when there is separation of charge imic band ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


