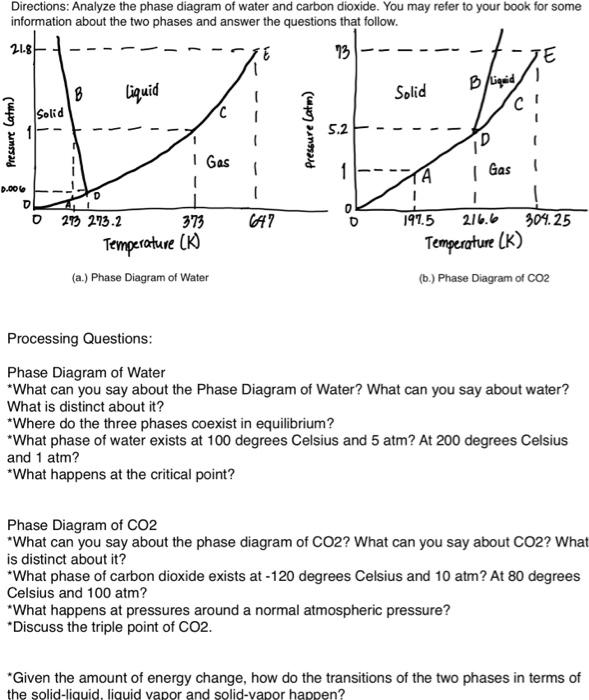
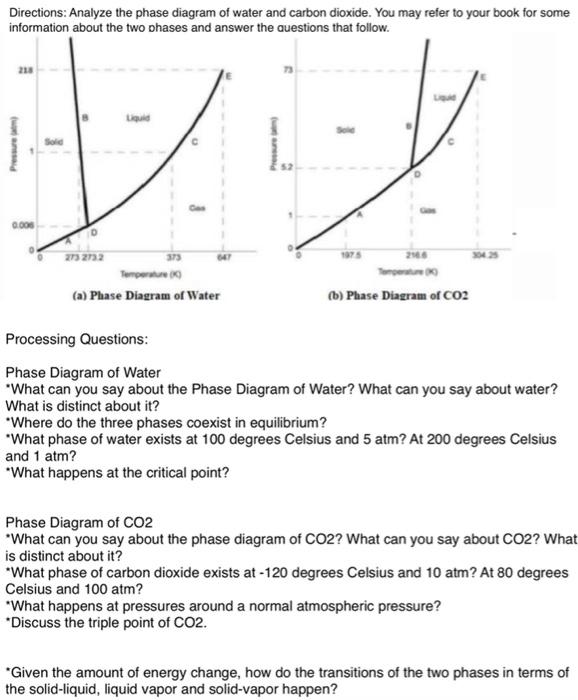
Directions: Analyze the phase diagram of water and carbon dioxide. You may refer to your book for some information about the two phases and answer the questions that follow. 21.84 13 B liquid Solid HA Bigad 1 Solid Pressure Catm) Pressure Catm) 5.2 1D I Gas 1 Gas D.006 D D 0 273 273.2 373 A7 0 O 1 197.5 216.6 304.25 Temperature (K) Temperature (K) (a.) Phase Diagram of Water (6.) Phase Diagram of CO2 Processing Questions: Phase Diagram of Water *What can you say about the Phase Diagram of Water? What can you say about water? What is distinct about it? *Where do the three phases coexist in equilibrium? *What phase of water exists at 100 degrees Celsius and 5 atm? At 200 degrees Celsius and 1 atm? *What happens at the critical point? Phase Diagram of CO2 *What can you say about the phase diagram of CO2? What can you say about CO2? What is distinct about it? *What phase of carbon dioxide exists at - 120 degrees Celsius and 10 atm? At 80 degrees Celsius and 100 atm? *What happens at pressures around a normal atmospheric pressure? *Discuss the triple point of CO2. "Given the amount of energy change, how do the transitions of the two phases in terms of the solid-liquid. liquid vapor and solid-vapor happen? Directions: Analyze the phase diagram of water and carbon dioxide. You may refer to your book for some information about the two phases and answer the questions that follow. 2. Liquid Sold Prowe C 0.000 273 2732 373 Tome (b) Phase Diagram of CO2 (a) Phase Diagram of Water Processing Questions: Phase Diagram of Water "What can you say about the Phase Diagram of Water? What can you say about water? What is distinct about it? "Where do the three phases coexist in equilibrium? "What phase of water exists at 100 degrees Celsius and 5 atm? At 200 degrees Celsius and 1 atm? "What happens at the critical point? Phase Diagram of CO2 "What can you say about the phase diagram of CO2? What can you say about CO2? What is distinct about it? "What phase of carbon dioxide exists at - 120 degrees Celsius and 10 atm? At 80 degrees Celsius and 100 atm? "What happens at pressures around a normal atmospheric pressure? *Discuss the triple point of CO2. "Given the amount of energy change, how do the transitions of the two phases in terms of the solid-liquid, liquid vapor and solid-vapor happen