Question
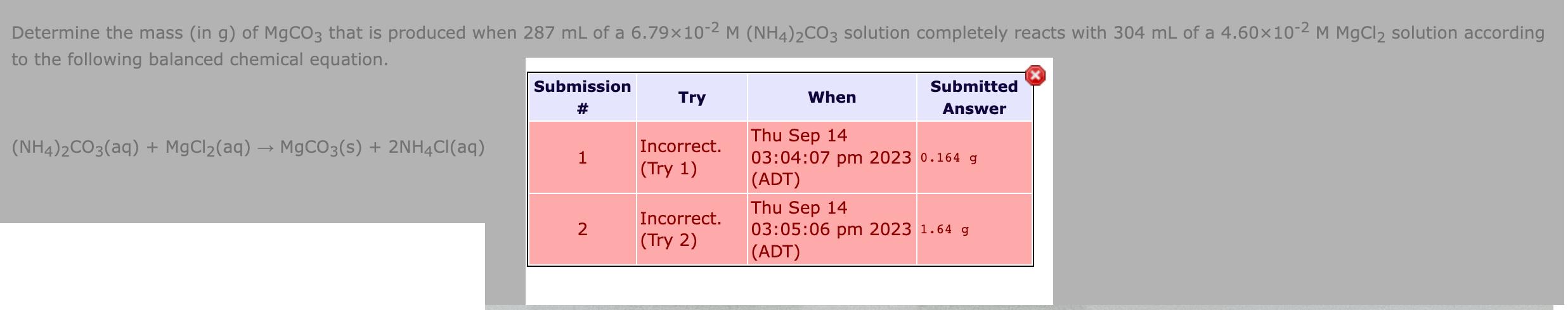
Determine the mass (in g) of MgCO3 that is produced when 287 mL of a 6.7910-2 M (NH4)2CO3 solution completely reacts with 304 mL
Determine the mass (in g) of MgCO3 that is produced when 287 mL of a 6.7910-2 M (NH4)2CO3 solution completely reacts with 304 mL of a 4.6010-2 M MgCl2 solution according to the following balanced chemical equation. Submission # Try When Submitted Answer Thu Sep 14 (NH4)2CO3(aq) + MgCl2(aq) MgCO3(s) + 2NH4Cl(aq) Incorrect. 1 (Try 1) 03:04:07 pm 2023 0.164 g (ADT) Thu Sep 14 2 Incorrect. (Try 2) 03:05:06 pm 2023 1.64 g (ADT)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Managerial Economics
Authors: Mark Hirschey
12th edition
9780324584844, 324588860, 324584849, 978-0324588866
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App