Question
Discussion and Conclusion Prompt Questions Do not number the answers in your typed discussion section, simply cover these topics in a logical way. 1. Discuss


Discussion and Conclusion Prompt Questions
Do not number the answers in your typed discussion section, simply cover these topics in a logical way.
1. Discuss the addition of calcium chloride drying agent after your extraction. Why was it important for your extract to be dry? What problems could have arisen had you not added drying agent?
2. With this procedure, do you think you are extracting all of the chemical compounds from the spinach leaves? Why or why not?
3. Discuss the separations of your compounds. Which spot/fraction likely corresponded to each compound (see page 11)? Comment on how you came to this conclusion. Include Rf values in your discussion.
4. Why did you use different solvent mixtures when running your column? Discuss what each solvent mixture did in terms of eluting your compounds and relate this to the chemical makeup of the compounds.
5. What is the purpose of evaporating your extraction solvent before separating the extracted mixture? Also, why is it important to not submerge your compound spot in the eluting solvent when running a TLC analysis?
6. How did you visualize each spot? Why did some compounds respond to UV light and others did not? Relate these observations to the chemical makeup of the compounds as well.
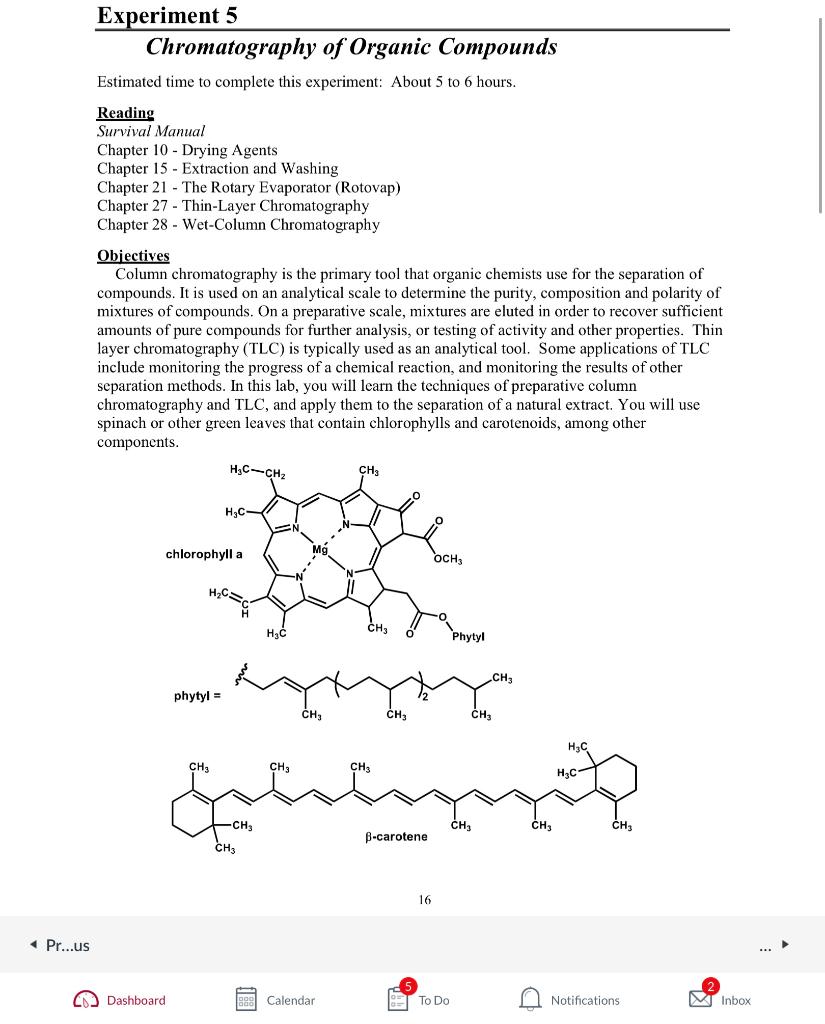
Objectives Column chromatography is the primary tool that organic chemists use for the separation of compounds. It is used on an analytical scale to determine the purity, composition and polarity of mixtures of compounds. On a preparative scale, mixtures are eluted in order to recover sufficient amounts of pure compounds for further analysis, or testing of activity and other properties. Thin layer chromatography (TLC) is typically used as an analytical tool. Some applications of TLC include monitoring the progress of a chemical reaction, and monitoring the results of other separation methods. In this lab, you will learn the techniques of preparative column chromatography and TLC, and apply them to the separation of a natural extract. You will use spinach or other green leaves that contain chlorophylls and carotenoids, among other components. phytyl = Extraction and Chromatography of Chlorophylls and Carotenoids 1.) Extraction. Weigh 10.0g of fresh spinach leaves (note actual weight). Tear the leaves into small pieces and place them in a mortar with 10mL of acetone. Grind with a pestle until the plant material has been broken into particles too small to be clearly seen. Filter the crude extract through a Buchner funnel. Rinse the mortar clean repeatedly with 1020mL portions of acetone up to a maximum total volume of 100mL. Pour the filtrate into a beaker and add 2-3 spoonfuls of calcium chloride. Swirl the solution a few times and gravity filter the solution into a 250-500 mL round-bottomed flask using fast filter paper. If an oil collects and clogs the filter toward the end, you can carefully decant this oil into the round-bottomed flask, combining it with the filtrate. Concentrate the solution on the rotovap to near dryness (35mL of liquid left). Your instructor will demonstrate the use of the rotovap. Partition your crude extract in a separatory funnel between ethyl acetate (EtOAc) and H2O(50mL each). (How will you do this?) Dry the EtOAc layer over calcium chloride and filter. Reserve a small amount of this solution for TLC analysis. Rotovap the EtOAc layer to dryness in a preweighed round-bottomed flask. Once dry, you can obtain a weight of the crude extract. 2.) Chromatography - Following the instructions on the next page, analyze your reserved EtOAc solution by TLC. This will give you a good idea of what to expect from the column chromatography. - Slurry-pack a silica gel flash column with hexane and 6g of silica gel (the instructor will demonstrate the slurry packing technique). You'll need to add a small ball of cotton at the bottom of the column prior to this. - Calculate your column bed volume based upon V=r2h,=3.14,r= internal radius of the column, h= height of the column of silica gel. - Redissolve your entire EtOAc extract in a minimal amount of hexane. - Load the whole sample onto column as depicted in Figure 6, being careful not to let the top of the column run dry. It is also important to keep the sample band as narrow as possible, which is aided by allowing the sample to descend into the packed column bed before adding the first eluting solvent (see step three of Figure 6). When adding solvents to the top of the column, try to do so with minimal disturbance of the silica gel layer. - Elute the column with the following solvent series (begin collecting when color starts eluting): TLC analysis of column fractions Use a large beaker with a watch glass cover as the developing chamber for the TLC plates. Add enough developing solvent to just cover the bottom of the beaker. TLC your column fractions on one plate if possible. In this lab you only need to spot fractions that visibly contain the compounds of interest, although normally you would spot all of them. Develop with a 3:2 solution of hexane-EtOAc. You can re-use this solvent once or twice if you keep it covered between analyses. In certain cases, you may need to re-run a TLC plate. For example, if the spots are too big or small, or if they run together or to one side. If they are too big you need to lower the concentration or spot less sample. If they are too small or are not visible, you need to spot more sample. If necessary, you may concentrate your samples on a hotplate in the hood, but do not overheat or evaporate to dryness as this can result in rapid decomposition. Draw in your notebook exactly what you see on each plate and indicate the following information in each drawing: - What compounds were spotted - The solvent used to develop the plate - The origin - The solvent front - The Rf values of all the spots seen - What methods were used to visualize the spots, and their appearance using each method Do not discard spotters after each use, keep about 510mL of ethanol nearby to rinse them after each use (take up the EtOH into the spotter, then blot it dry onto a paper towel...repeat). You shouldn't need more than one or two spotters for the whole lab period. Save the spotters in a safe place in your lab drawer. Record your results, noting the colors of each spot and circling the spots lightly with a pencil. Then place the entire plate in one of the iodine chambers provided. Observe what happens after 5-10 minutes in the chamber. Alternatively, use a UV lamp to visualize any additional spots. Objectives Column chromatography is the primary tool that organic chemists use for the separation of compounds. It is used on an analytical scale to determine the purity, composition and polarity of mixtures of compounds. On a preparative scale, mixtures are eluted in order to recover sufficient amounts of pure compounds for further analysis, or testing of activity and other properties. Thin layer chromatography (TLC) is typically used as an analytical tool. Some applications of TLC include monitoring the progress of a chemical reaction, and monitoring the results of other separation methods. In this lab, you will learn the techniques of preparative column chromatography and TLC, and apply them to the separation of a natural extract. You will use spinach or other green leaves that contain chlorophylls and carotenoids, among other components. phytyl = Extraction and Chromatography of Chlorophylls and Carotenoids 1.) Extraction. Weigh 10.0g of fresh spinach leaves (note actual weight). Tear the leaves into small pieces and place them in a mortar with 10mL of acetone. Grind with a pestle until the plant material has been broken into particles too small to be clearly seen. Filter the crude extract through a Buchner funnel. Rinse the mortar clean repeatedly with 1020mL portions of acetone up to a maximum total volume of 100mL. Pour the filtrate into a beaker and add 2-3 spoonfuls of calcium chloride. Swirl the solution a few times and gravity filter the solution into a 250-500 mL round-bottomed flask using fast filter paper. If an oil collects and clogs the filter toward the end, you can carefully decant this oil into the round-bottomed flask, combining it with the filtrate. Concentrate the solution on the rotovap to near dryness (35mL of liquid left). Your instructor will demonstrate the use of the rotovap. Partition your crude extract in a separatory funnel between ethyl acetate (EtOAc) and H2O(50mL each). (How will you do this?) Dry the EtOAc layer over calcium chloride and filter. Reserve a small amount of this solution for TLC analysis. Rotovap the EtOAc layer to dryness in a preweighed round-bottomed flask. Once dry, you can obtain a weight of the crude extract. 2.) Chromatography - Following the instructions on the next page, analyze your reserved EtOAc solution by TLC. This will give you a good idea of what to expect from the column chromatography. - Slurry-pack a silica gel flash column with hexane and 6g of silica gel (the instructor will demonstrate the slurry packing technique). You'll need to add a small ball of cotton at the bottom of the column prior to this. - Calculate your column bed volume based upon V=r2h,=3.14,r= internal radius of the column, h= height of the column of silica gel. - Redissolve your entire EtOAc extract in a minimal amount of hexane. - Load the whole sample onto column as depicted in Figure 6, being careful not to let the top of the column run dry. It is also important to keep the sample band as narrow as possible, which is aided by allowing the sample to descend into the packed column bed before adding the first eluting solvent (see step three of Figure 6). When adding solvents to the top of the column, try to do so with minimal disturbance of the silica gel layer. - Elute the column with the following solvent series (begin collecting when color starts eluting): TLC analysis of column fractions Use a large beaker with a watch glass cover as the developing chamber for the TLC plates. Add enough developing solvent to just cover the bottom of the beaker. TLC your column fractions on one plate if possible. In this lab you only need to spot fractions that visibly contain the compounds of interest, although normally you would spot all of them. Develop with a 3:2 solution of hexane-EtOAc. You can re-use this solvent once or twice if you keep it covered between analyses. In certain cases, you may need to re-run a TLC plate. For example, if the spots are too big or small, or if they run together or to one side. If they are too big you need to lower the concentration or spot less sample. If they are too small or are not visible, you need to spot more sample. If necessary, you may concentrate your samples on a hotplate in the hood, but do not overheat or evaporate to dryness as this can result in rapid decomposition. Draw in your notebook exactly what you see on each plate and indicate the following information in each drawing: - What compounds were spotted - The solvent used to develop the plate - The origin - The solvent front - The Rf values of all the spots seen - What methods were used to visualize the spots, and their appearance using each method Do not discard spotters after each use, keep about 510mL of ethanol nearby to rinse them after each use (take up the EtOH into the spotter, then blot it dry onto a paper towel...repeat). You shouldn't need more than one or two spotters for the whole lab period. Save the spotters in a safe place in your lab drawer. Record your results, noting the colors of each spot and circling the spots lightly with a pencil. Then place the entire plate in one of the iodine chambers provided. Observe what happens after 5-10 minutes in the chamber. Alternatively, use a UV lamp to visualize any additional spotsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


