Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Disinfection is one of the major steps in the drinking water treatment process. Depending on the source of water being treated, there is high

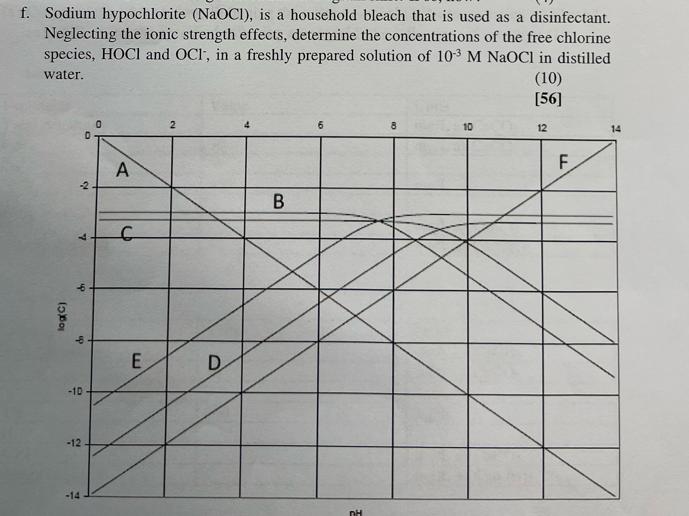
Disinfection is one of the major steps in the drinking water treatment process. Depending on the source of water being treated, there is high likelihood of producing disinfection by- products (DBP) when chlorine is used as disinfectant. a. Describe how the formation of disinfection by products takes place, discuss which water sources are more likely to present this problem and propose methods that can be used to mitigate DBP formation. (10) b. Chlorine dioxide has been proposed as a possible solution in the prevention of DBP formation. Discuss mechanism by which chlorine dioxide imparts disinfection in water. Briefly discuss the advantages and disadvantages associated with the use of chlorine dioxide. NB. You are encouraged to use and cite journal articles for this question. (10) c. In order to obtain a free residual of chlorine of 0.5 mg/L, a dose of 10 mg/L is added to a 36400 m/day water treatment plant. NB It is common practice to apply Cl:NH3 dosage ratios of 3:1 (i) Calculate the amount of Cl2 and NH3 required to achieve this. (8) (ii) Explain why it is sometimes necessary to add ammonia during the disinfection process and what is the reasoning behind using a 3:1 Cl2:NH3 dosage ratio (6) Chlorine usage in a 20 000 m/day treatment plant is 8 kg. The residual after 10 min contact time is 0.15 mg/L. What is the chlorine demand of the water? (4) d. e. The graph below shows the log-concentration diagram of a system containing 5 x 104 M NH3 and an unknown amount of Sodium hypochlorite. Determine. i. The unknown species A to F ii. The sodium concentration iii. iv. (6) (2) The pH (2) In a similar system, the NH3 is substituted with 2.5 x10-4 M NH4C1 and 2.5 x104 M NH3 Will the log-concentration diagram shift? If so, how? (4) f. Sodium hypochlorite (NaOCI), is a household bleach that is used as a disinfectant. Neglecting the ionic strength effects, determine the concentrations of the free chlorine species, HOCI and OCI, in a freshly prepared solution of 10 M NaOCl in distilled water. log(C) 7 -2 ch ip D -10- -12 -14 0 A C E 2 D B 6 nH 8 10 (10) [56] 12 F. 14
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


